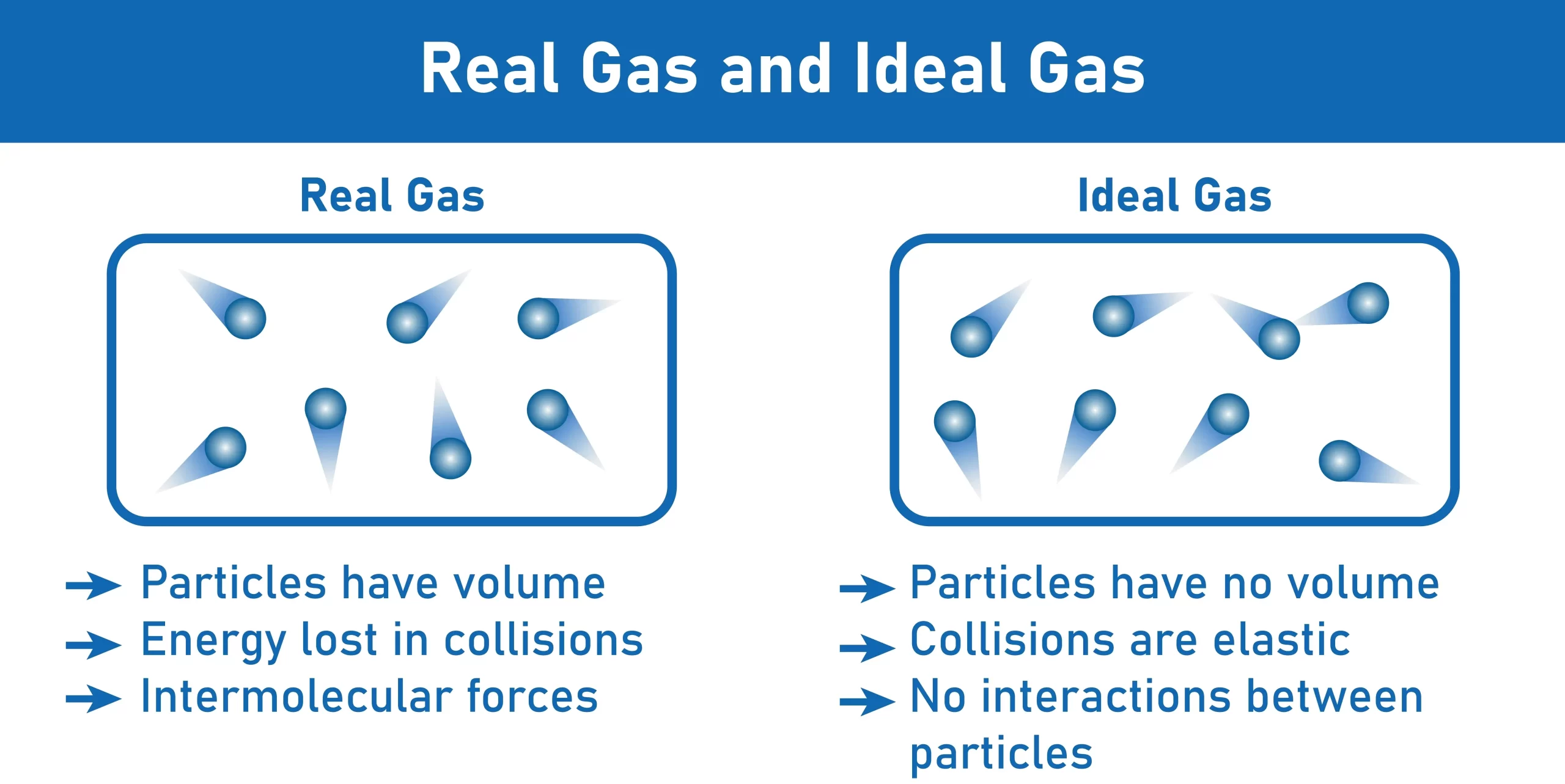
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the …
The empirical relationships among the volume, the temperature, the pressure, and the amount of a gas can be combined into the ideal gas law, PV = nRT. The proportionality constant, R, is called the gas constant. The ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the kinetic molecular theory of gases. Standard temperature and pressure (STP) is 0°C and 1 atm.
The Ideal Gas Law Boundless Chemistry

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases

Chemistry 103-Principles of Chemistry PDF, PDF, Chemical Compounds
What volume will 2.5mol of a gas occupy at 283K and at a pressure of 300torr under ideal conditions? (He also says 3 significant digits and ' (R=62.36L * torr/ (mol *

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases
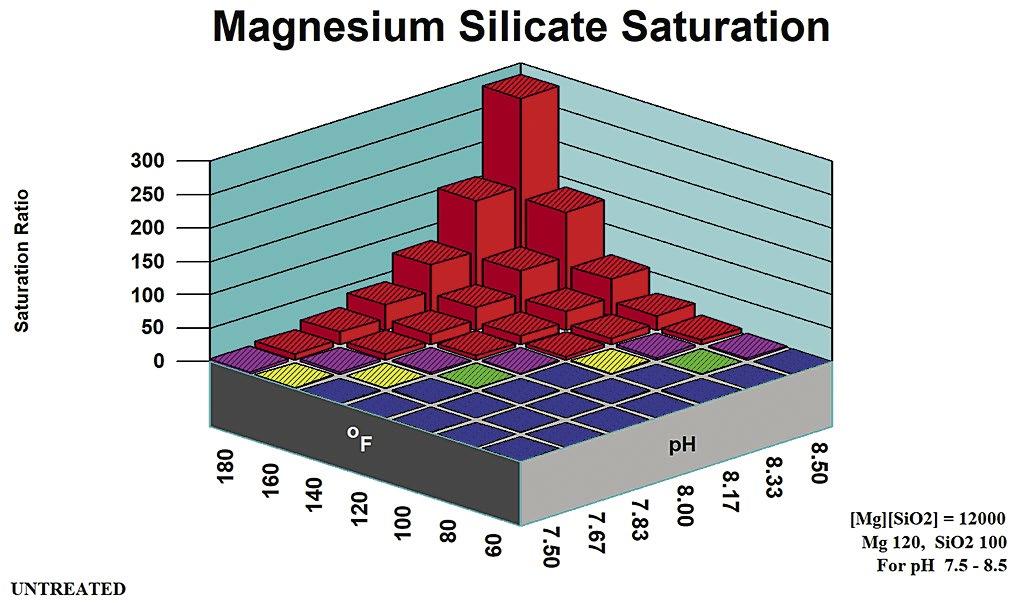
AWT Winter 2023 Analyst by Association of Water Technologies - Issuu
1. An unspecified ideal gas at 10°C and 100kPa occupies a volume of 2.5 m³. (a) How many moles does this gas have? (b) If the pressure and temperature are raised three

Gas « KaiserScience

The Ideal Gas Law - Chemistry LibreTexts, PDF, Gases