2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1

At 300 K 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would

Board Que 2023, PDF, Coordination Complex
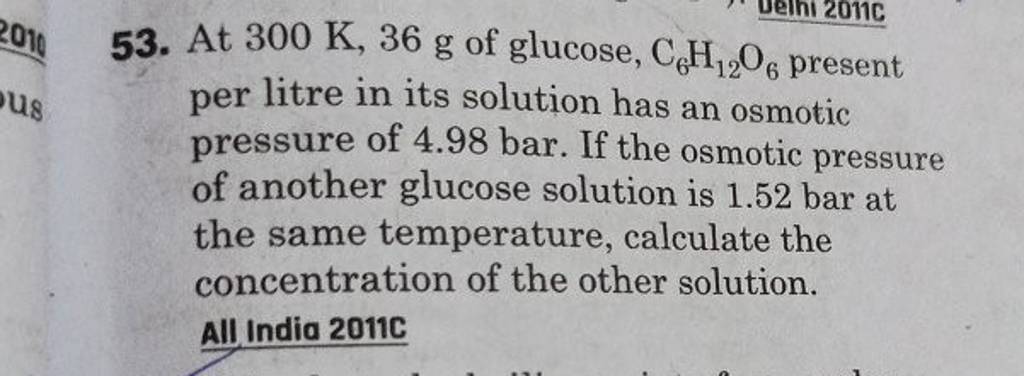
53. At 300 K,36 g of glucose, C6H12O6 present per litre in its solutio..

V4OQ2BFgESYNczkHR2GX.pdf
How many grams of glucose, C6H12O6, are necessary to prepare 598 ml of solution with concentration of 0.72 molar? - Quora

At S.T.P., the density of nitrogen monoxide is - (1) 3.0 GL-1 (2) 30 GL-1 (3) 1.34 L-1 (4)2.68 gL-1
NCERT Ebook for Solutions - Solutions - Chapter 2 - NCERT Chemistry - XII
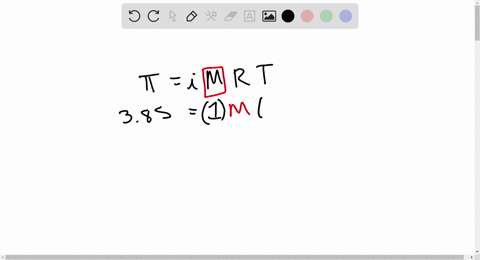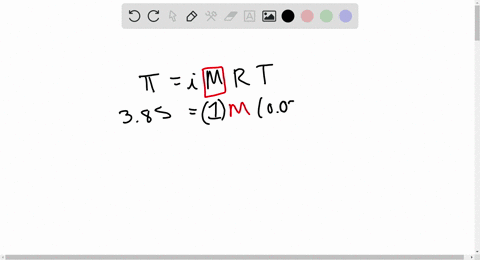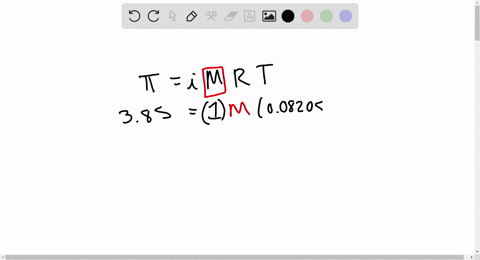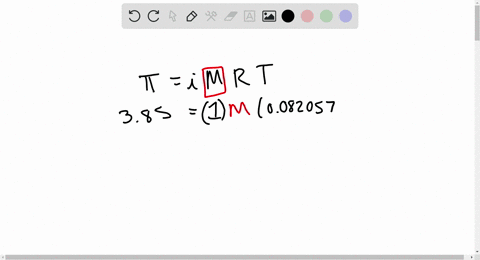
⏩SOLVED:At 300 K, 36 g of glucose present per litre in its…

At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the

NCERT Solutions Class 12 Chemistry Chapter 1 - Solutions





