20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

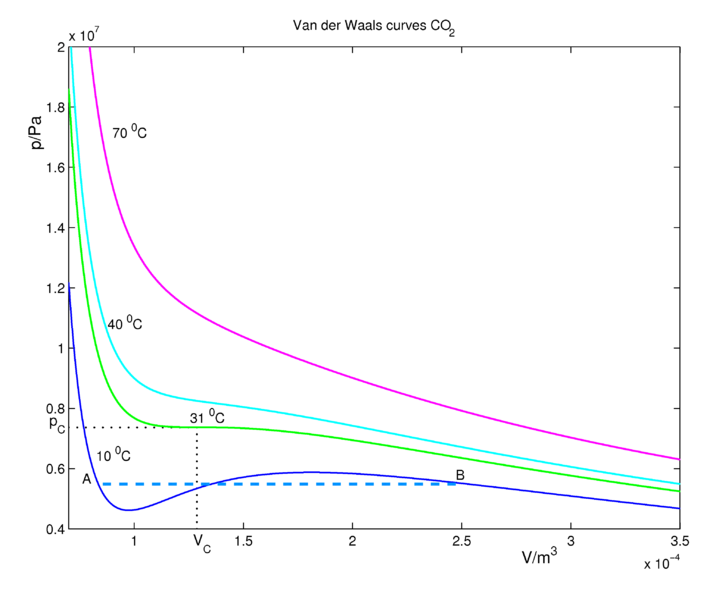
Real Gases and Compressibility Factor

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Compressibility factor Z versus ρ ¯ for the n = 4 fluid. The plot

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
16.3: A Cubic Equation of State - Chemistry LibreTexts

Fluids, Free Full-Text
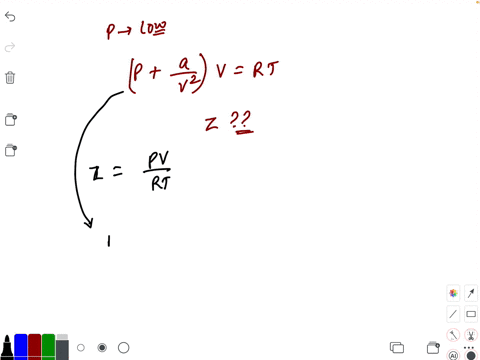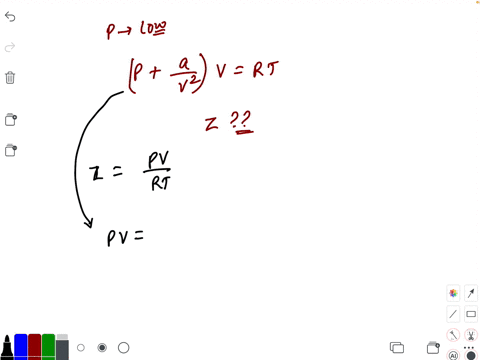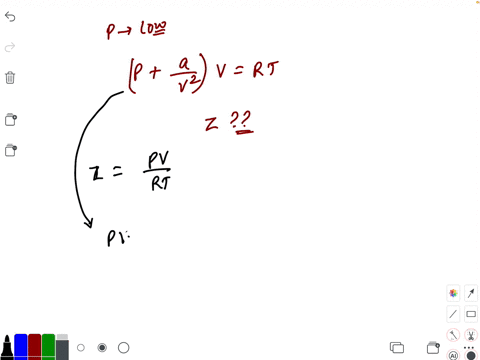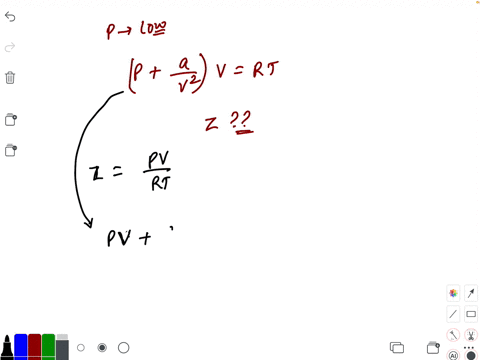
⏩SOLVED:At low pressures, van der Waals' equation is written as…

⏩SOLVED:For a van der Waals gas with given values of a and b,…
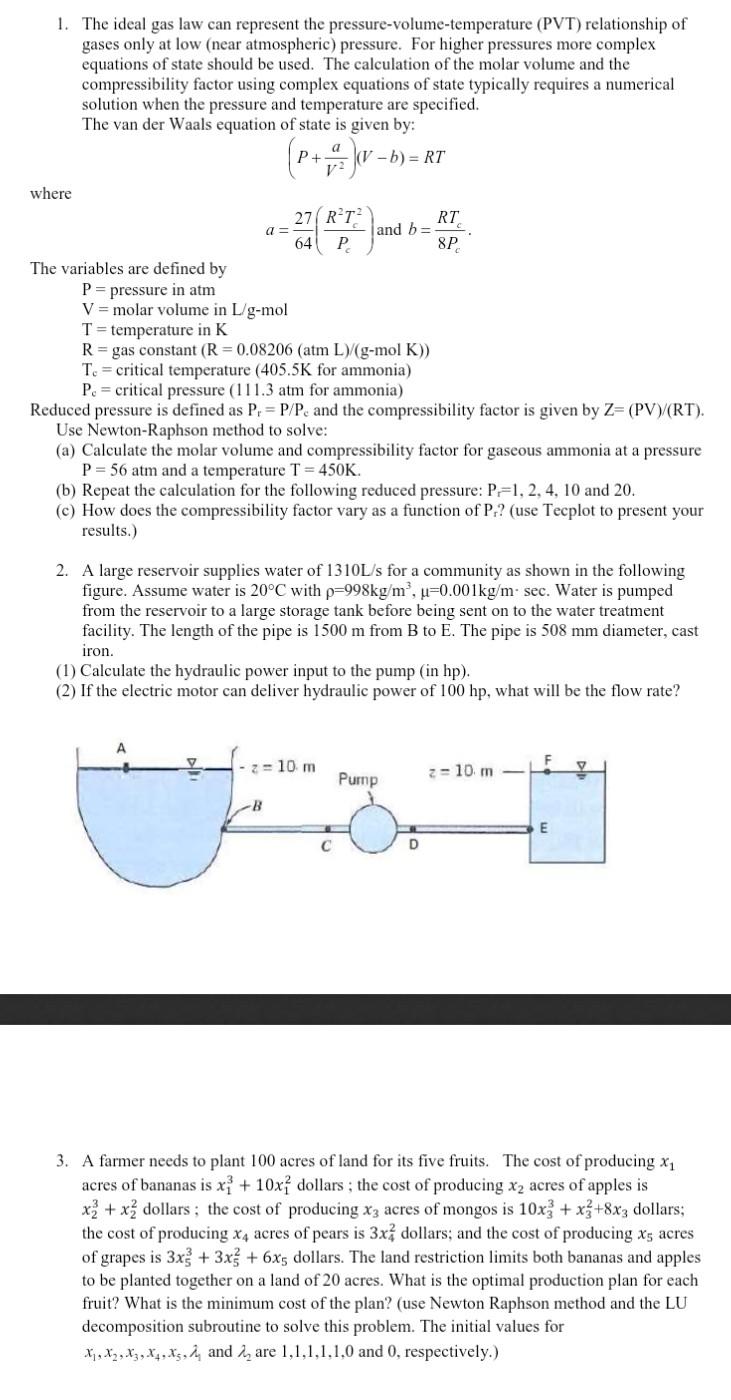
Solved Can you solve the problem and add fortran code for

At a high pressure, the compressibility factor (Z) of a real gas is us
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as







