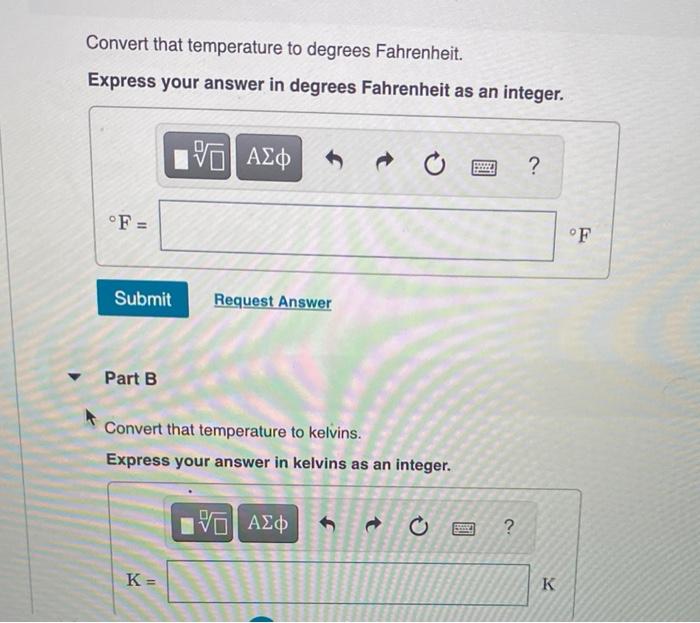

Freezing point of an aqueous solution is `-0.186^(@)C`. Elevation of boiling point of the
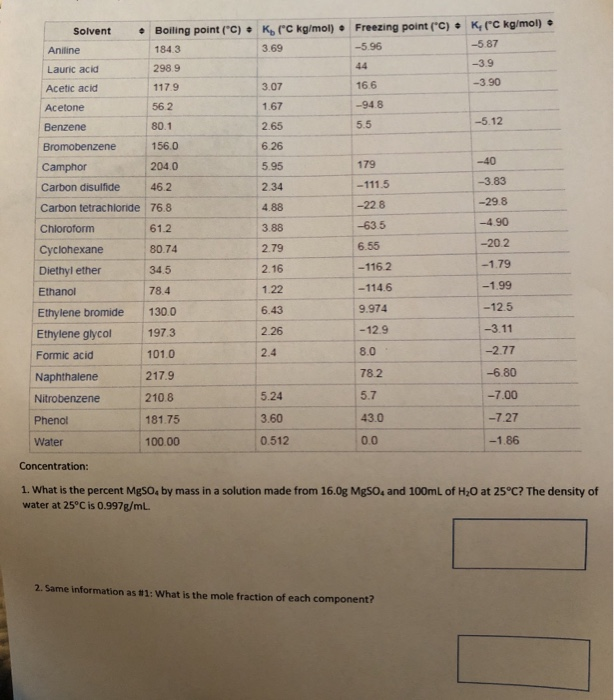
Solved Solvent • Boiling point (C) K (C kg/mol) Freezing

Thermo problem set no. 2
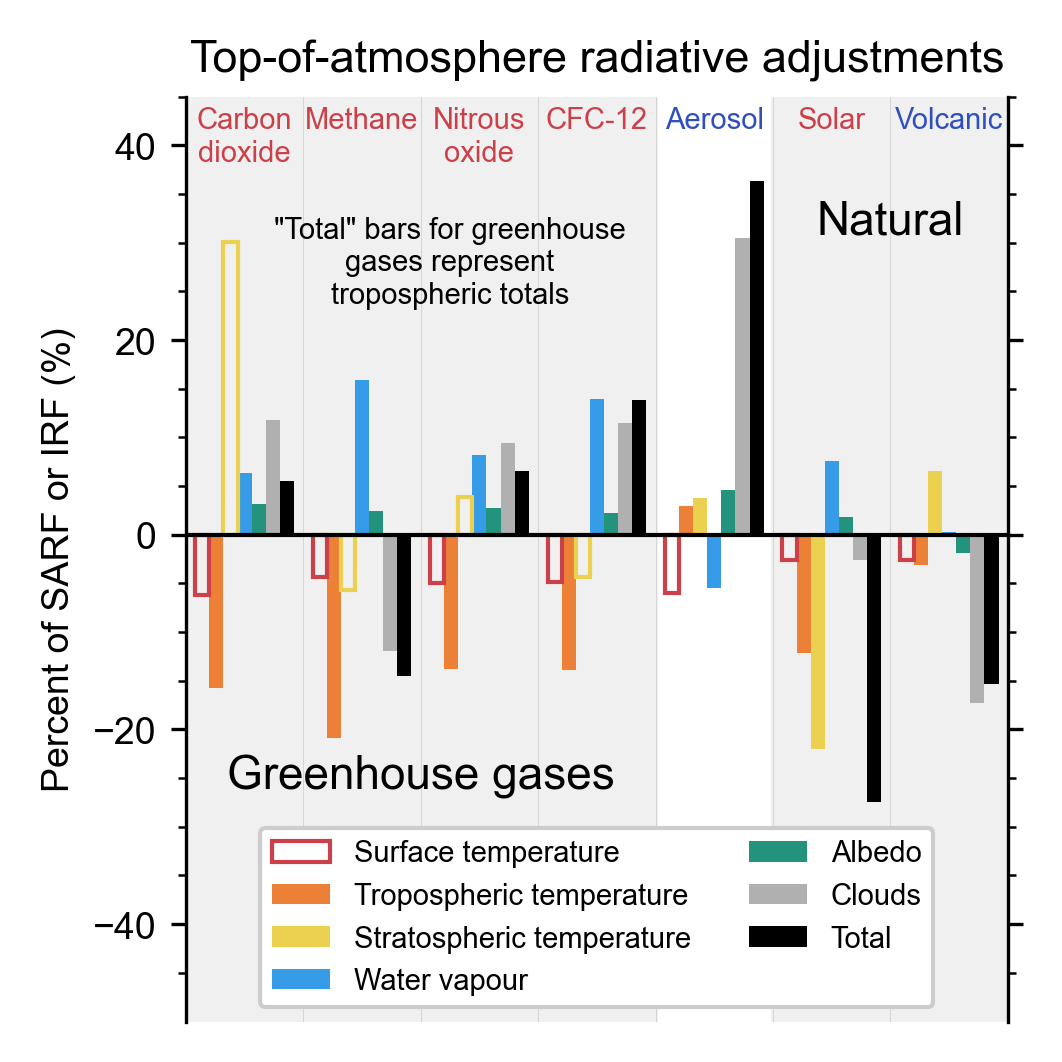
Chapter 7: The Earth's Energy Budget, Climate Feedbacks, and Climate Sensitivity

Find the expected freezing point of a water solution that co
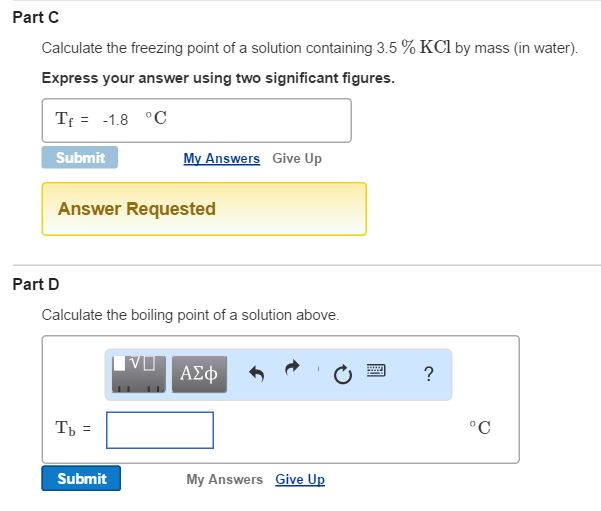
Solved Calculate the freezing point of a solution containing

Freezing of Lakes

SOLVED: The experimentally measured freezing point of a 1.40 m aqueous solution of AlCl3 is -8.33°C. The freezing point depression constant for water is Kf = 1.86°C/m. Assume the freezing point of

SOLVED: Calculate the boiling point in degrees Celsius of a 3.69 m solution of KI in water. Remember that KI is an electrolyte. The normal boiling point of pure water is 100.0°C.

The freezing point of a solution containing `50 cm^(3)` of ethylene glycol in `50 g` of water is

56 Willies Lane, Fox Point — For Sale @ $499,900







