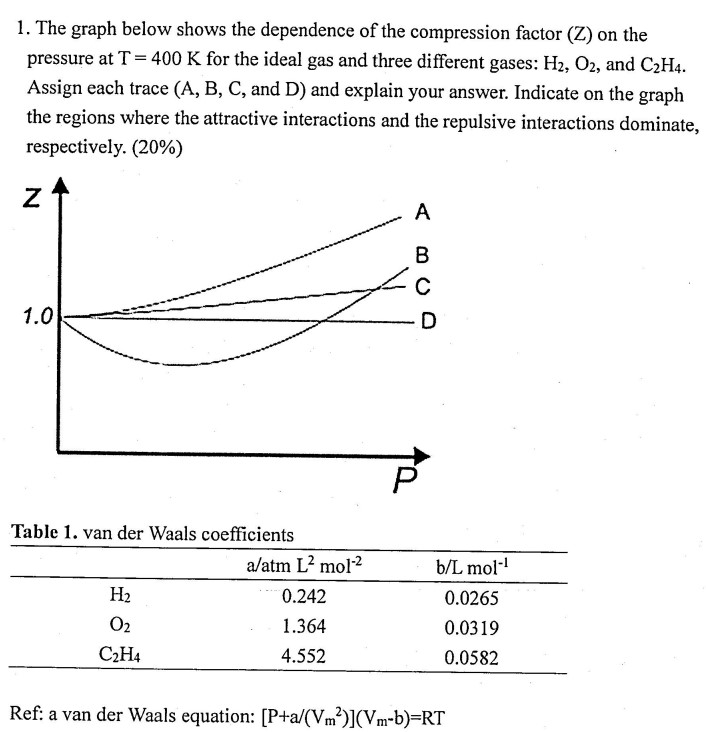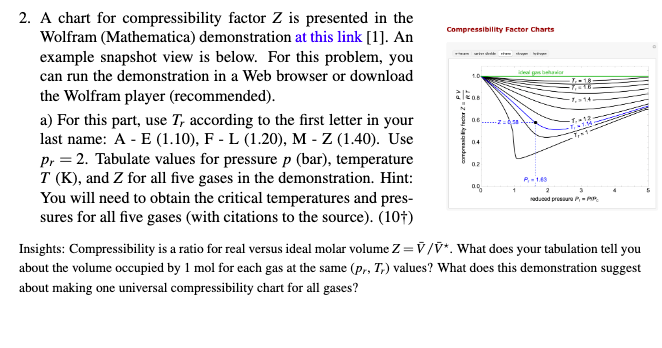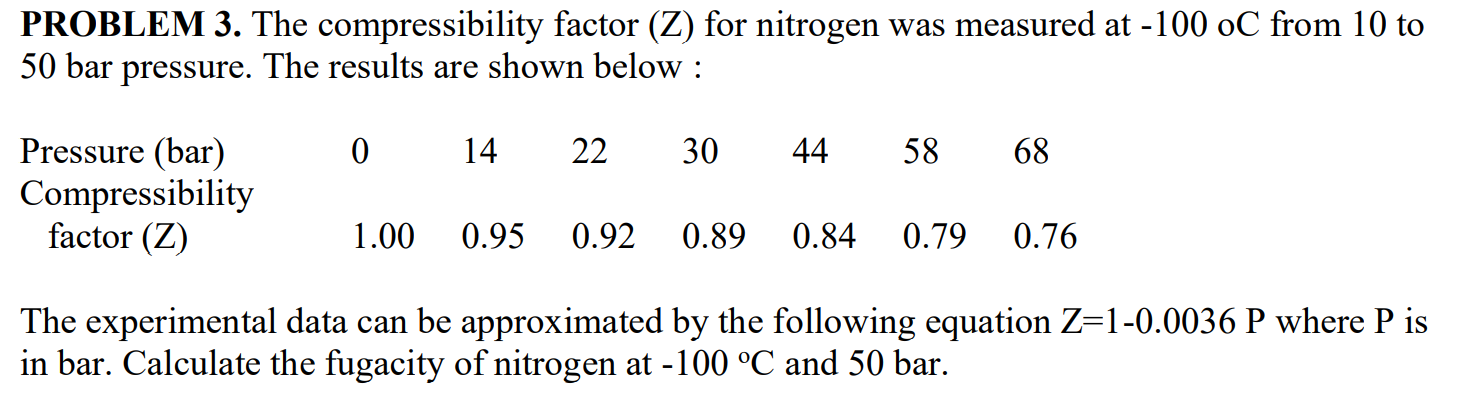
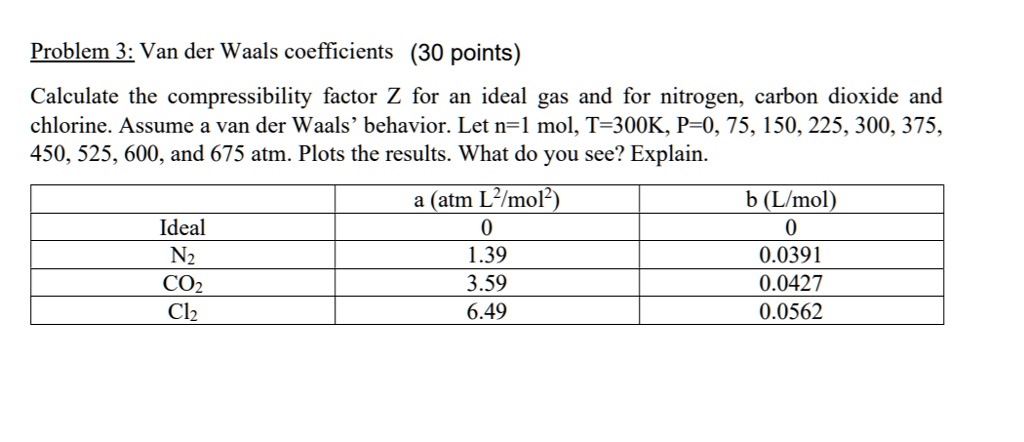
SOLVED: Problem 3: Van der Waals coefficients (30 points) Calculate the compressibility factor Z for an ideal gas and for nitrogen, carbon dioxide, and chlorine. Assume a van der Waals' behavior: Let

Plot of Hall and Yarborough z factor chart with convergence problem

Answered: (a)Using the compressibility chart,…

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Superheated water vapor is at 40 Mpa, 550 degree Celsius. Can you consider this vapor as an ideal gas? Support your answer by calculating the compressibility factor. a) What is the specific

Non-Ideal Gas Behavior Chemistry: Atoms First
Solved Problem 3 (35 marks): Calculate the compressibility

Compressibility factor - Wikipedia
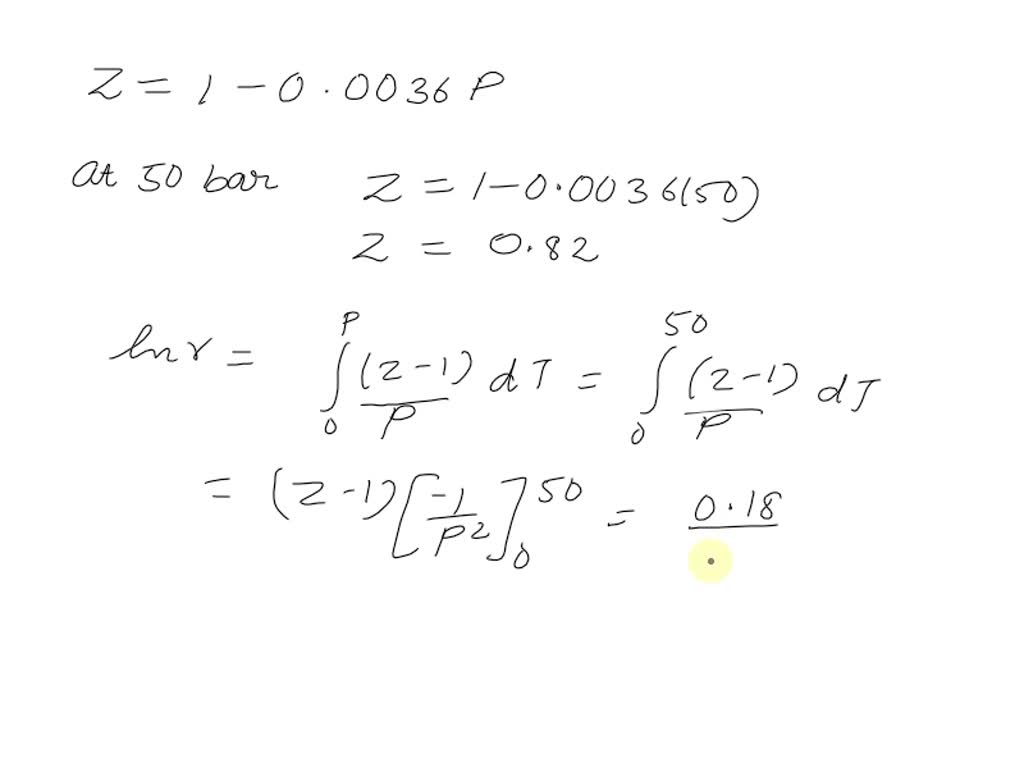
SOLVED: PROBLEM 3: The compressibility factor (Z) for nitrogen was measured at -100 °C from 10 to 50 bar pressure. The results are shown below: Pressure (bar) Compressibility factor (Z) 0 14