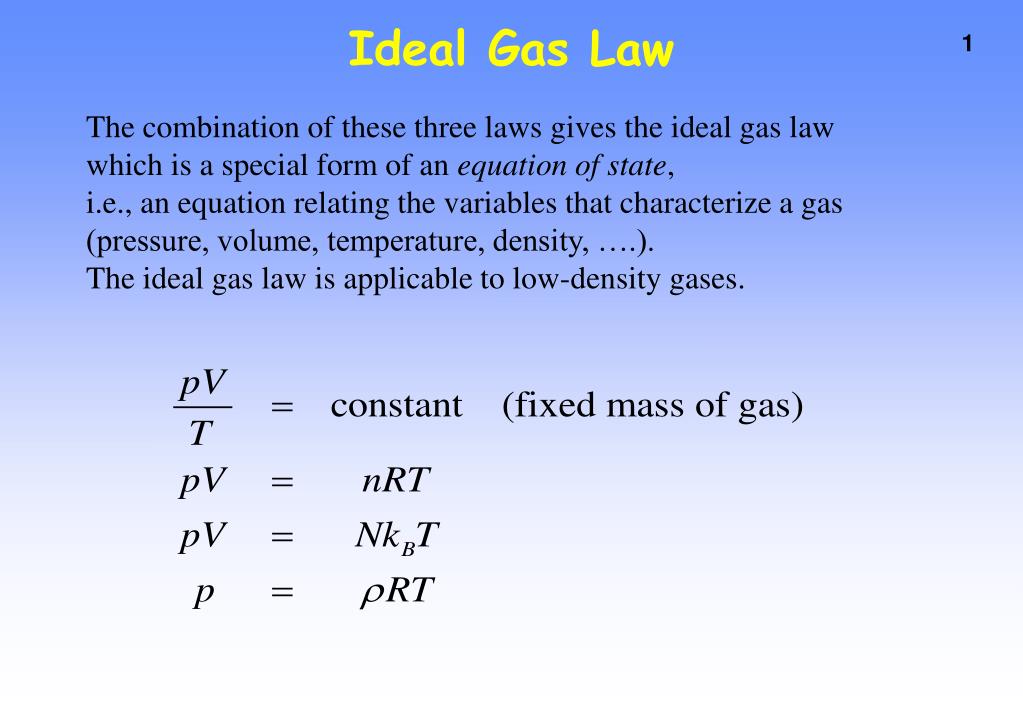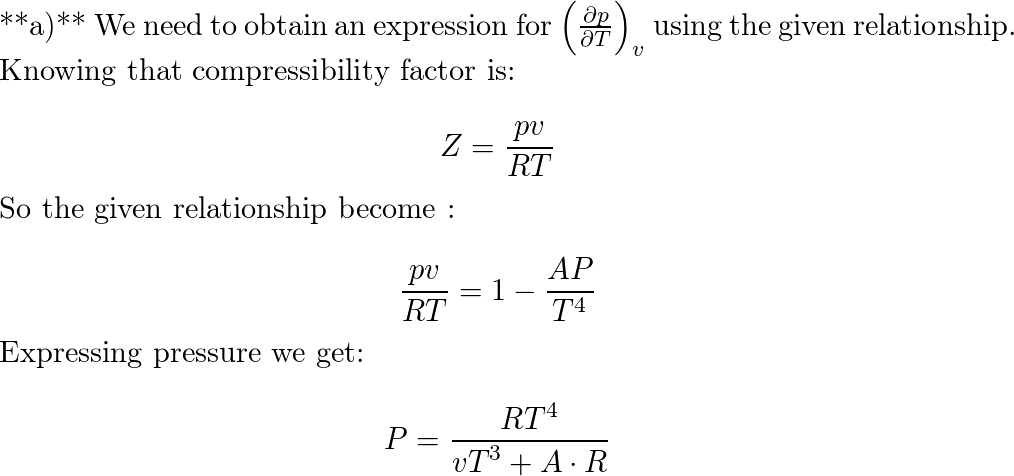

Phase Changes
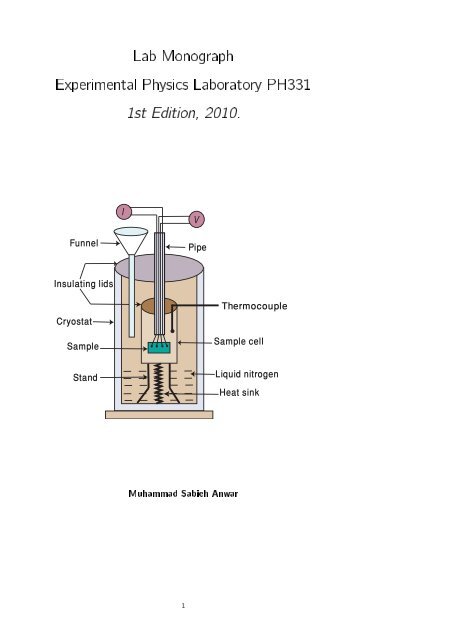
Lab Manuals in Compiled Form - Experimental Physics
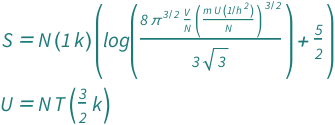
Sackur–Tetrode Equation Using Internal Energy
Gas solubility in a liquid increases with decreasing temperature, thereby decreasing the partial pressure of that gas above the liquid. So how does Henry's law still stand? As the partial pressure decreases

Van der Waals' Equation of State for a Non-Ideal Gas - Wolfram Demonstrations Project
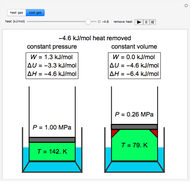
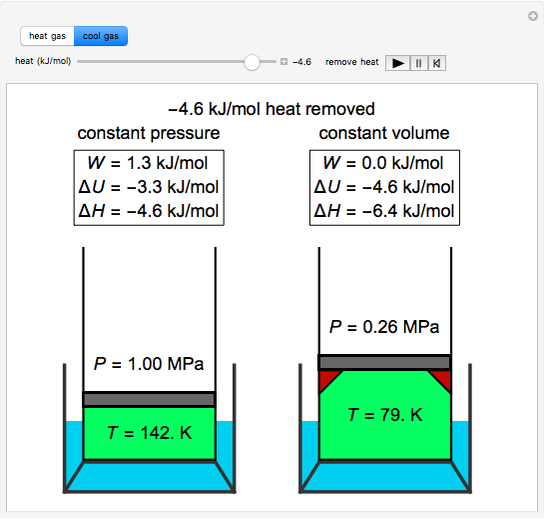
Temperature Changes in an Ideal Gas - Wolfram Demonstrations Project
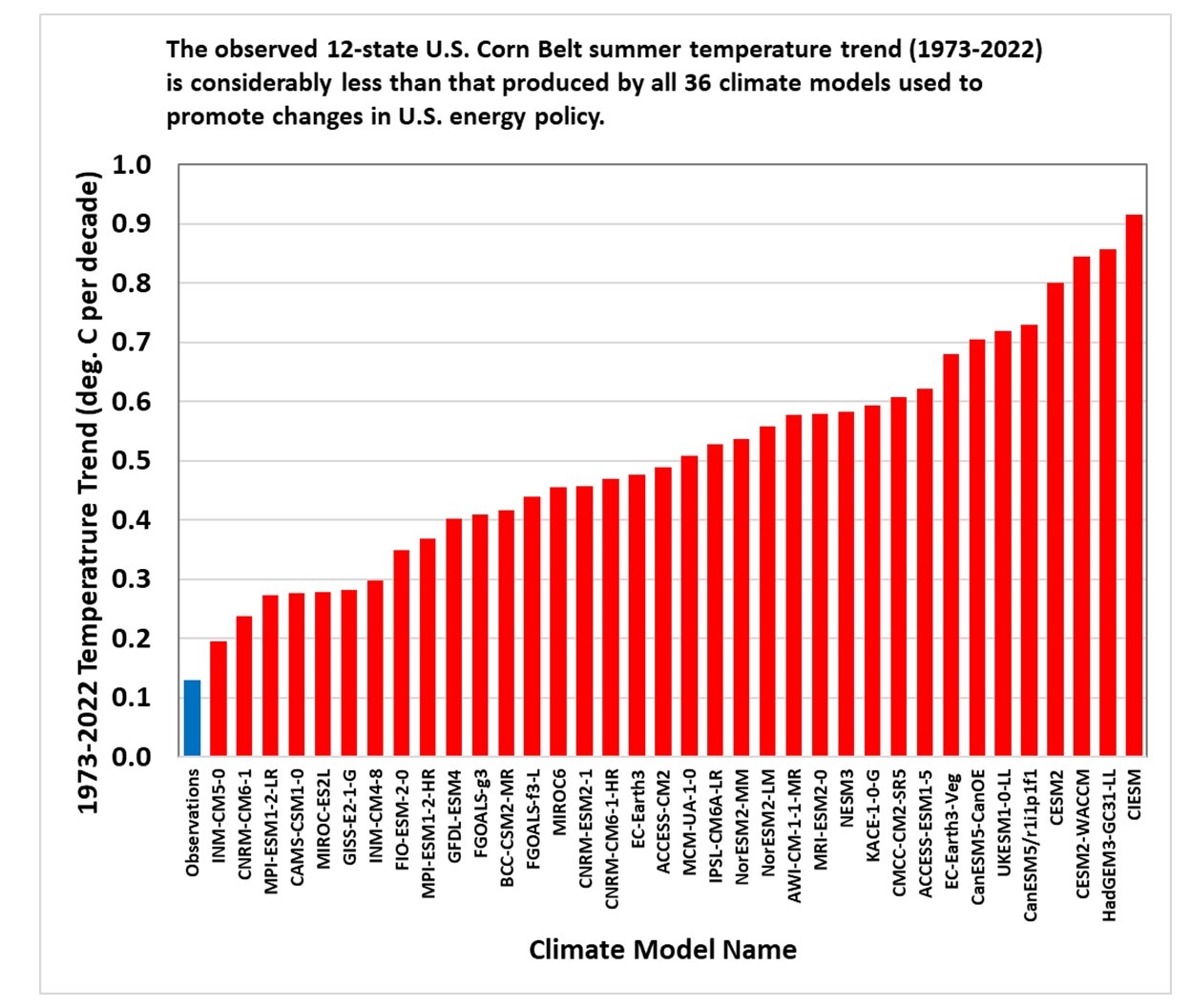
Epic Fail in America's Heartland: Climate Models Greatly Overestimate Corn Belt Warming « Roy Spencer, PhD

The optimal tuning, within carbon limits, of thermal mass in naturally ventilated buildings - ScienceDirect

Influence of regeneration conditions on cyclic CO2 adsorption on NaA zeolite at high pressures - ScienceDirect
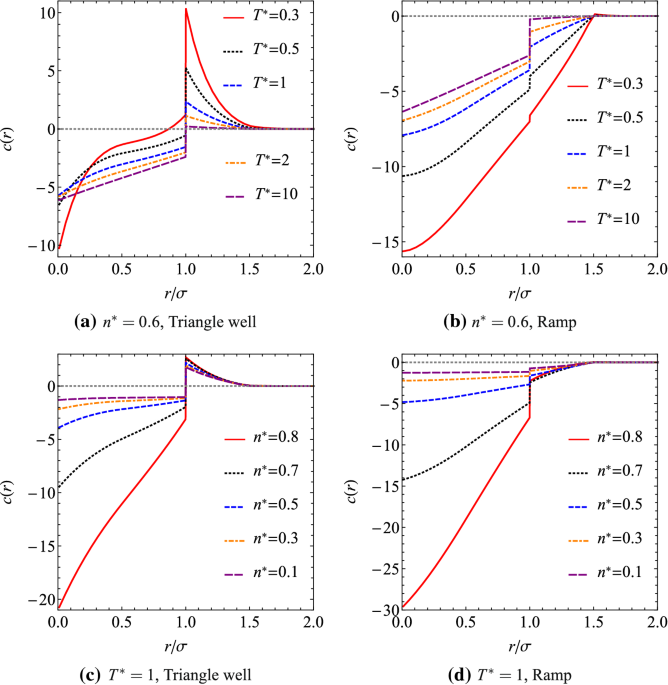
Triangle-Well and Ramp Interactions in One-Dimensional Fluids: A Fully Analytic Exact Solution
Compute-to-Learn Demos and Publications