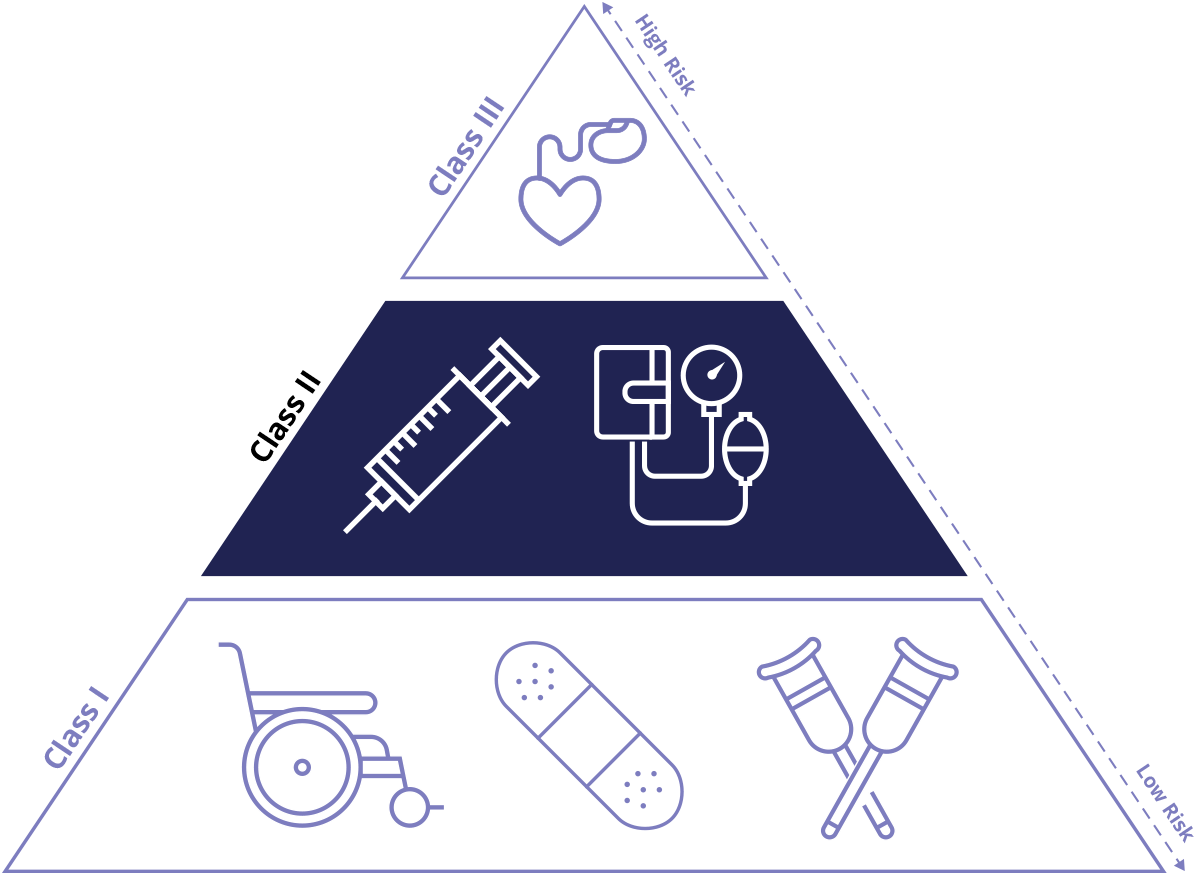
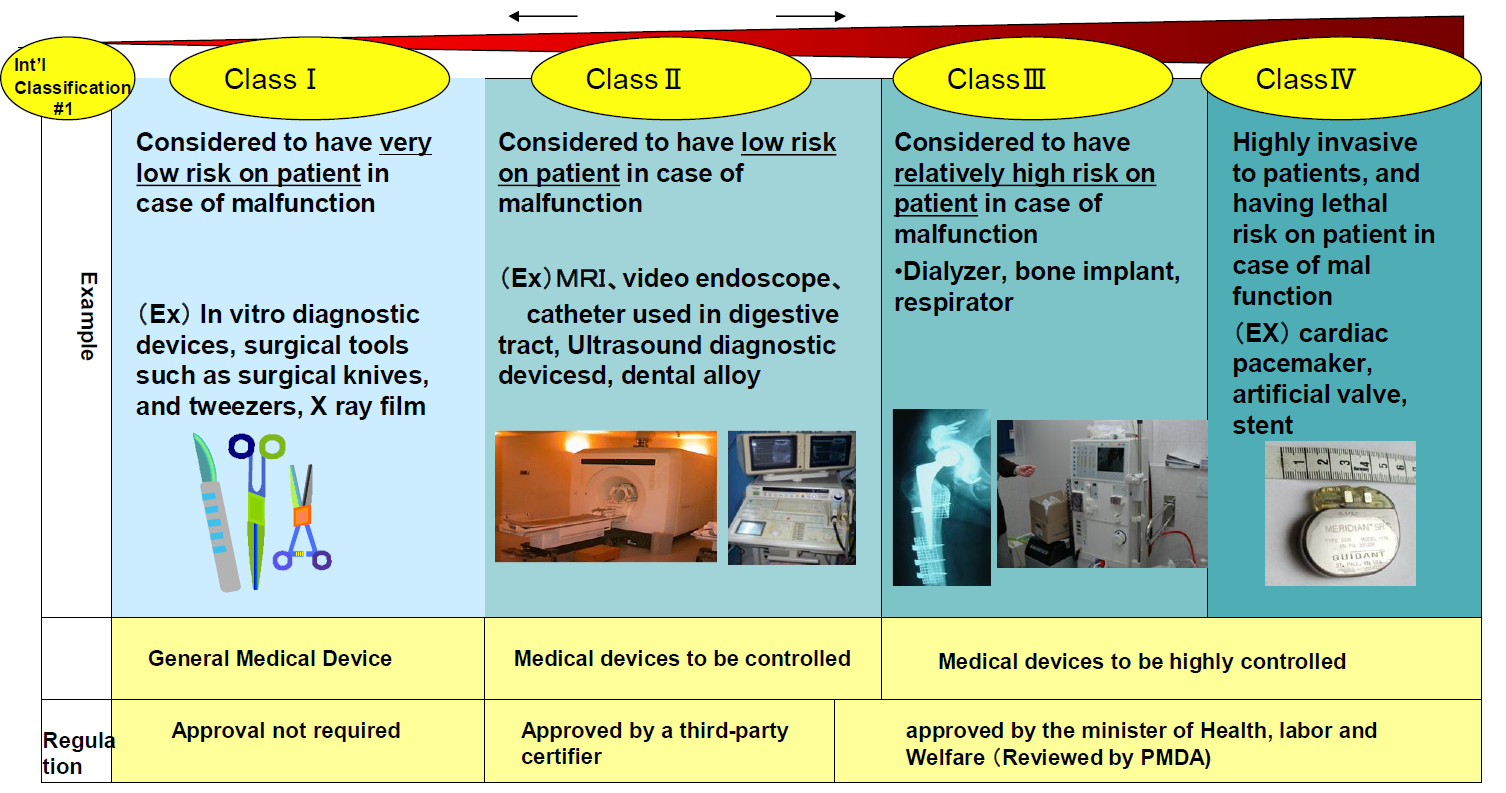
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.
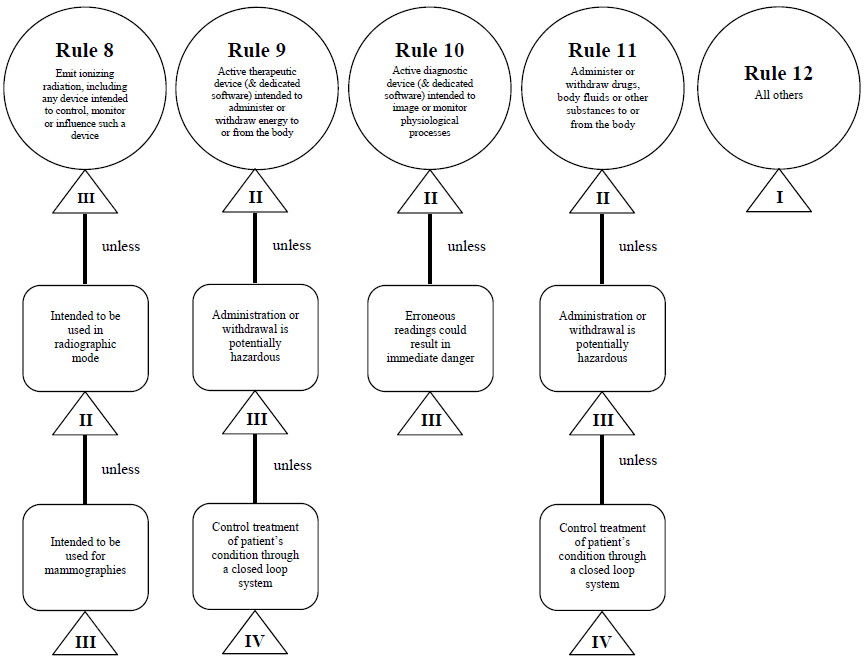
Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)

Entering the US Market: Medical Devices - ppt video online download

Never accept the mark of the beast

Medical device regulations, classification & submissions
Class II vs. Class III medical devices

FDA Class II medical devices
Interoperability standards for medical device integration in the OR and issues relating to international approval procedures (part 4) - ISCASBlog

Classify Your Medical Device

All Class 1 Medical Device Manufacturers Must Meet These Specific EU MDR Requirements – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
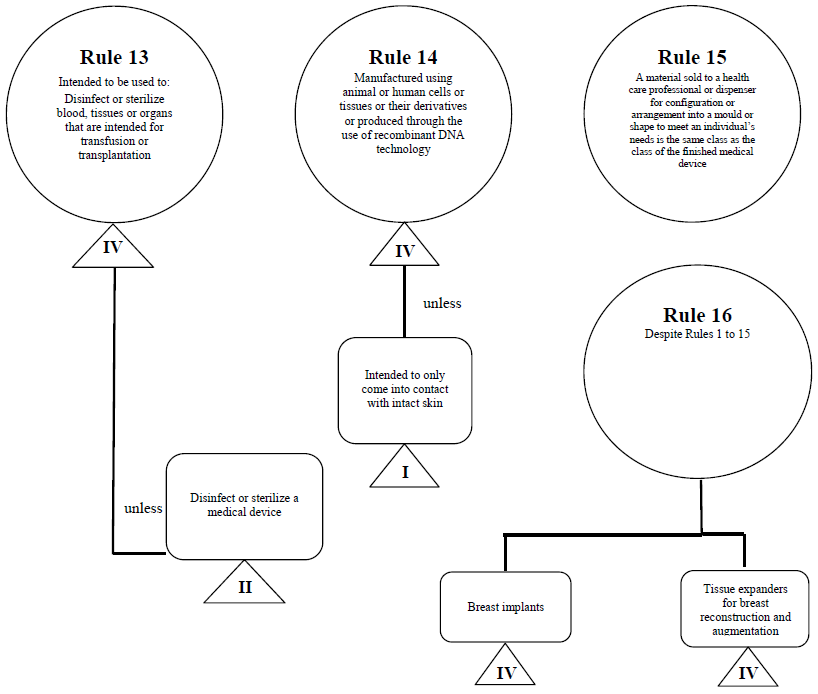
Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)