
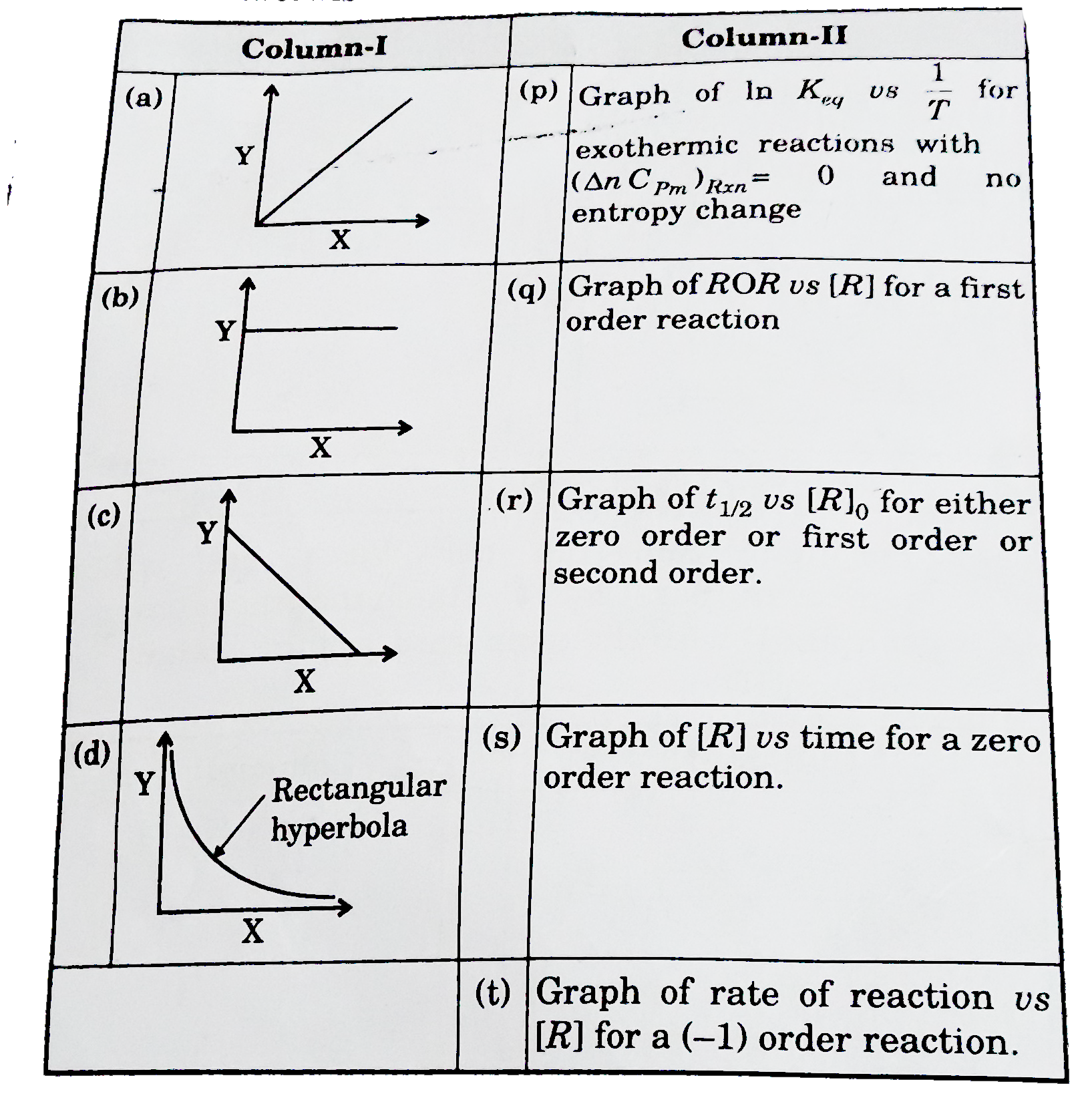
Share your videos with friends, family and the world

PDF) Critical State Behavior of Van der Waal gases Conformation to Nelson Obert Characteristics
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

Chapter 8 - -~. . 8.1 Demonstrate the law of corresponding states by writing the van der Waals equation in terms of the reduced variables. Calculate
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora
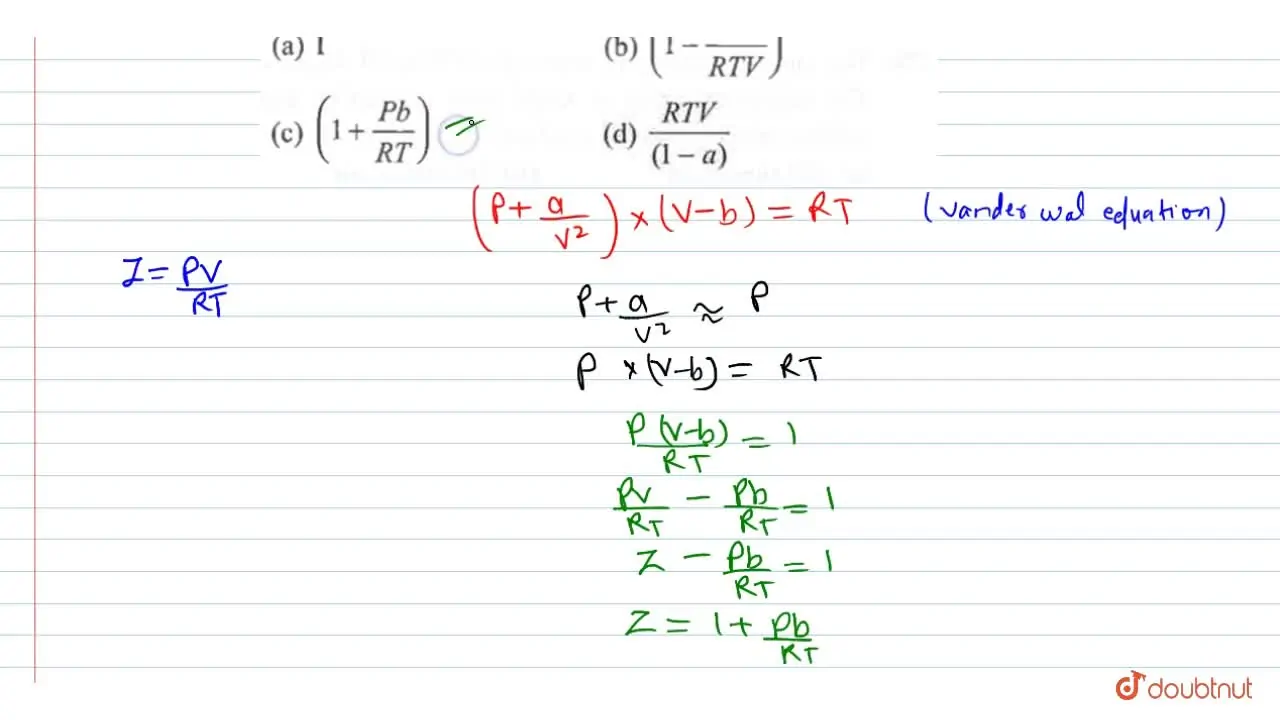
Compressibility factor for H(2) behaving as real gas is

JEE: Van der Waals Equation, Chemistry By Unacademy
1.5 Real Gases and the Virial Equation - Mail
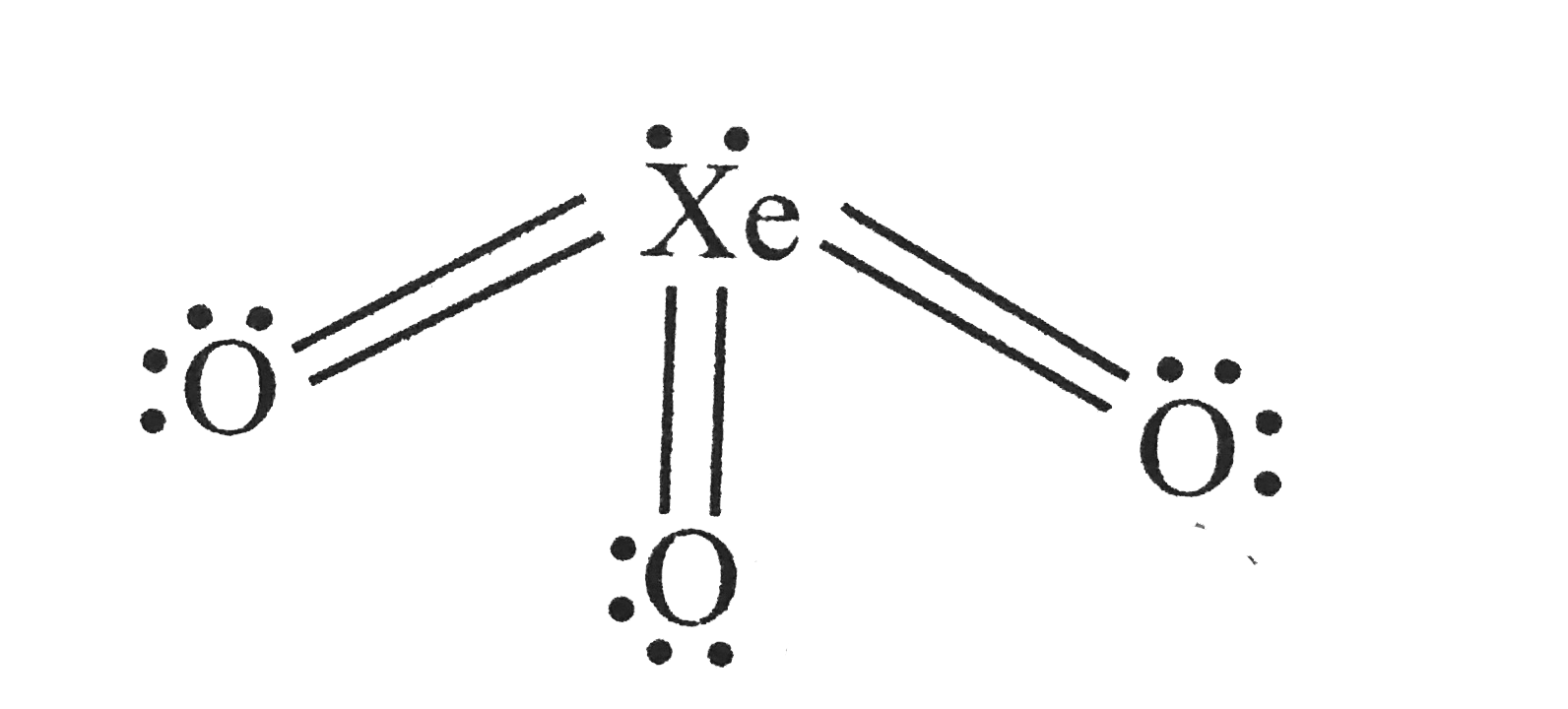
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions

Determine Compressibility of Gases
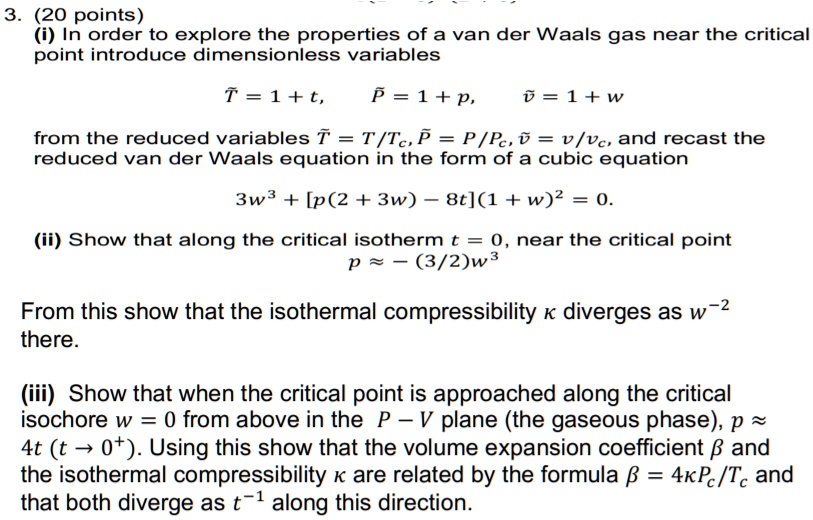
SOLVED: (i) In order to explore the properties of a van der Waals gas near the critical point, introduce dimensionless variables: T = 1 + t, P = 1 + p, V =

Notes - CHE 2203 Chemical Thermodynamics and Thermochemistry-1, PDF, Gases

Compressibility factor (Z) for a van der Waals real gas at critical po







