lt;p>Using the correct media is critical to ensure microbiological quality. Explore a portfolio of culture media and substances for sample preparation, microbial enumeration tests, and tests for specified microorganisms.</p>

PDF) Quality Control of Non-Sterile Drug Product According to United States' Pharmacopeia Instruction
A Full Spectrum Of Compliant Ready-To-Use Mycoplasma Media With An Extended Shelf Life

PDF) Microbiological quality control of some non-sterile preparations commonly used in Pakistan

Microbial limit test

How To Establish Growth Promotion Tests For Pharmaceutical Culture Media
What is environmental monitoring in pharmaceutical industry

Sterility Test, how to perform Sterility Test in the best way?

Major dii erences between Sterile and Non-Sterile Drugs and Biologics

General Purpose Media : Types, Composition, Preparation, and Uses : A Comprehensive Guide

Microbial Culture Media Preparation – FC-BIOS SDN BHD

/rmbdata/userfiles/test_m

Making MS-Based Glycan Analysis Easier

Sterility Testing
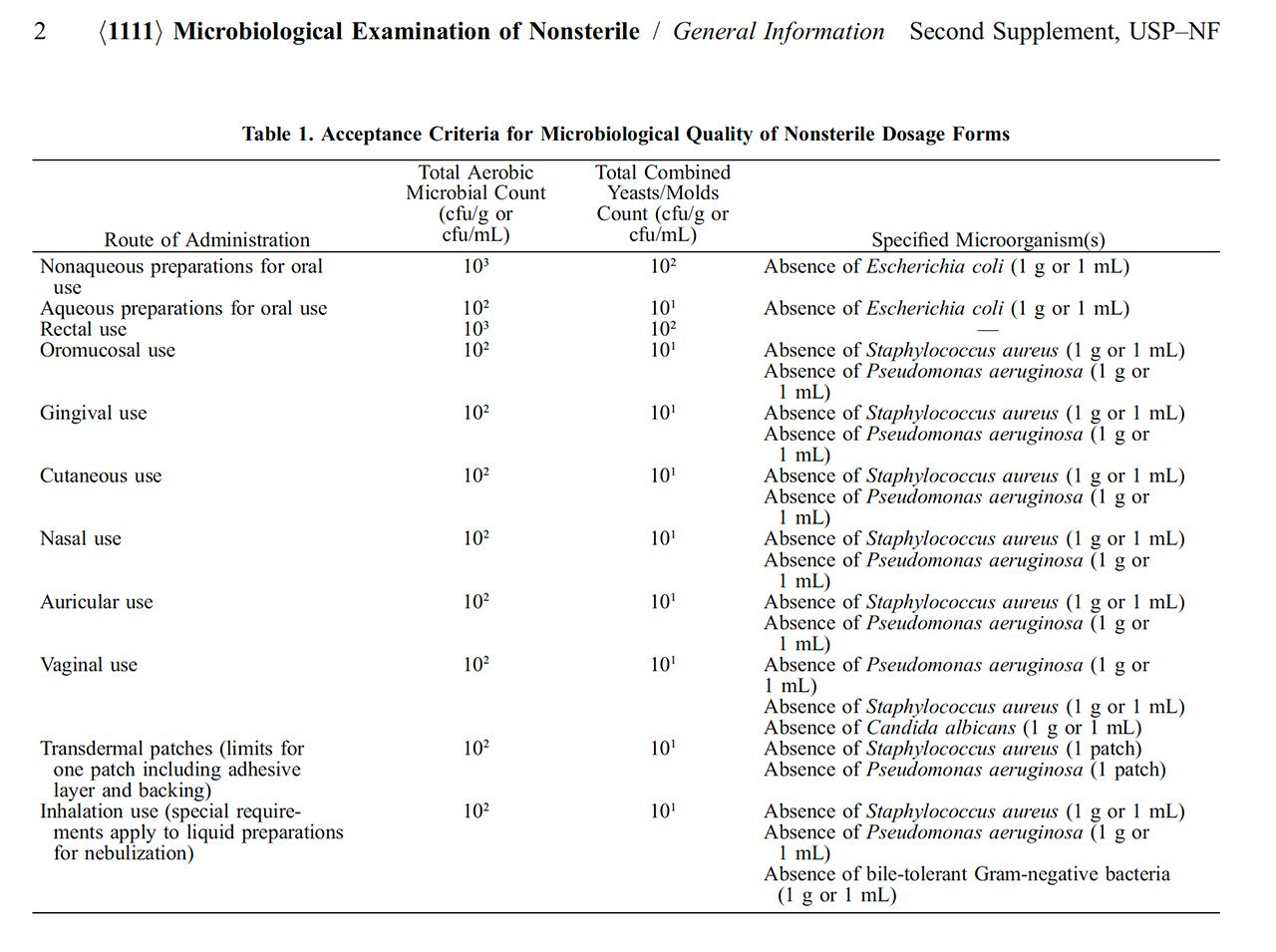
Refining Microbiological Control for Non-Sterile Products

Testing of Purified Water, Raw Materials, In-Process Samples and Finished Non-Sterile Products