:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.

How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry
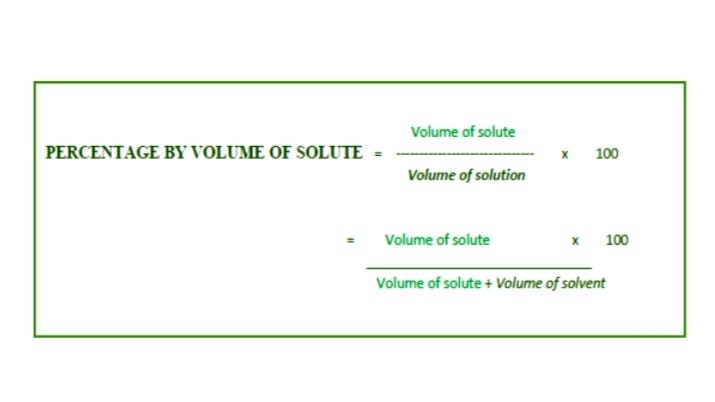
Molarity, Molality, Normality, Part per million (ppm) and other basic terms of Concentration solution with definition & formula, Chemistry Basic, 02, by Amrita Shetty
What is the normality of solution obtained by mixing 100 ml of 0.2 M H2SO4 with 100 ml of 0.2 M NaOH? - Quora

Normality calculation - example problems

Normality Calculation - Chemistry

Calculate the normality of HCl solution whose 500 ml is utillised to neutralise the 1500 ml of
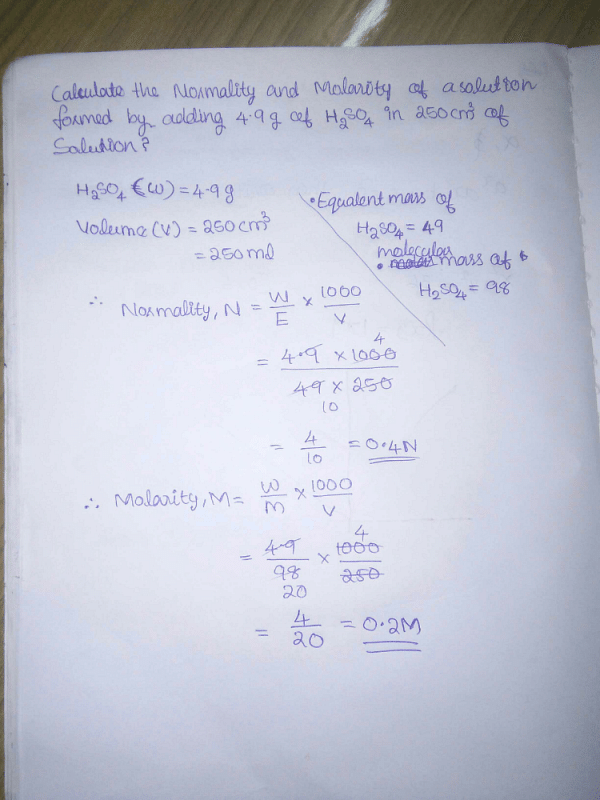
Calculate the Normality and Molarity of a solution formed by adding 4.9g of H2SO4 in 250cm^3 of solution? - EduRev Class 12 Question

SOLUTION: Difference between molarity molality and normality and how to calculate them - Studypool

Normality - Formula, Definition, Examples, Problems

How to Calculate Normality: 4 Steps (with Pictures) - wikiHow

Normality Calculation - Chemistry







