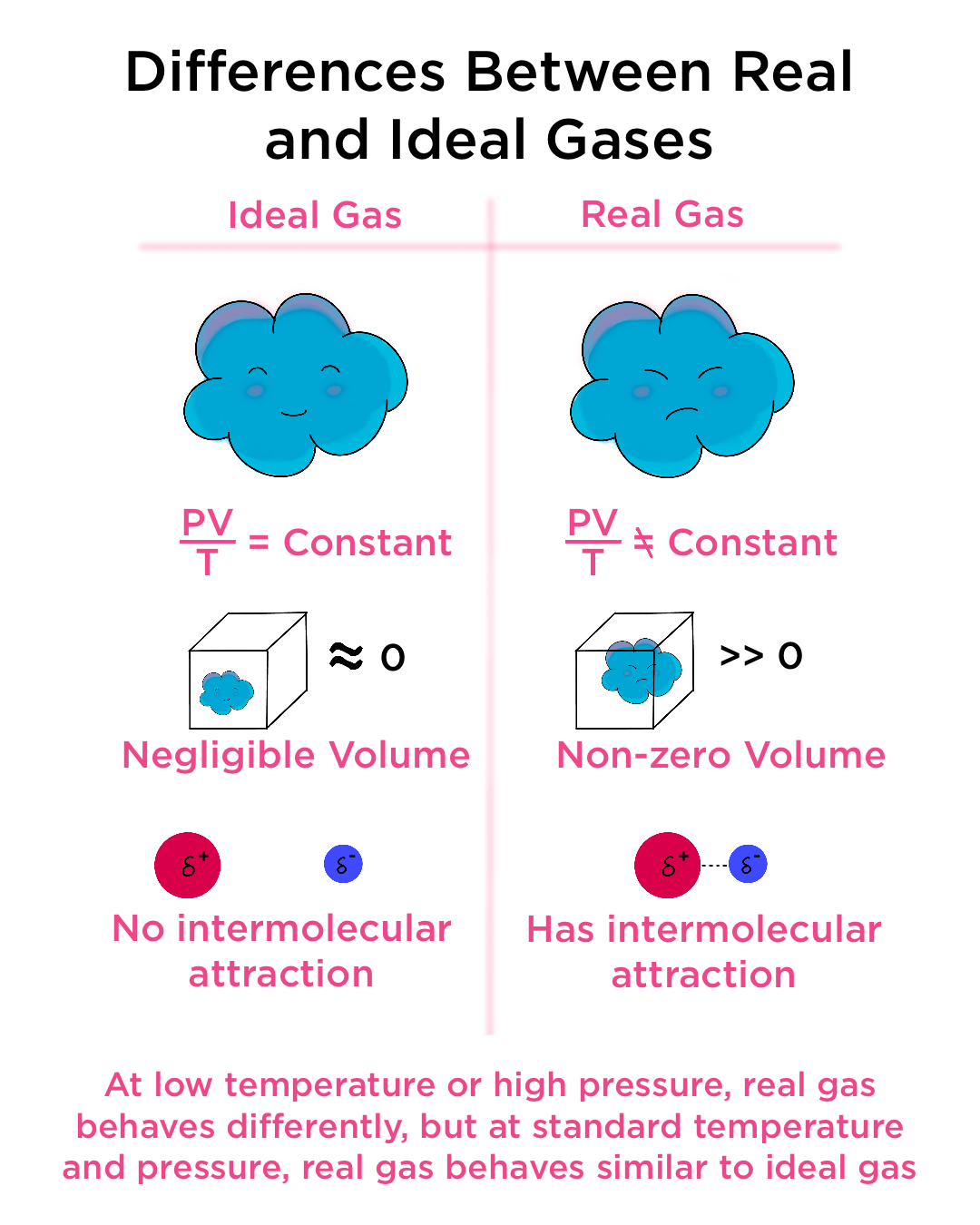
Ideal gas law, relation between the pressure P, volume V, and temperature T of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In such a case, all gases obey an equation of state known as the ideal gas law: PV =

How to Calculate a Final Temperature Using the Ideal Gas Law

Ideal Gas Law: Calculation of Universal Gas Constant, General Chemistry
6.3: Combining the Gas Laws: The Ideal Gas Equation and the
Ideal gases and the ideal gas law: pV = nRT

The ideal gas law (PV = nRT) Intermolecular forces and
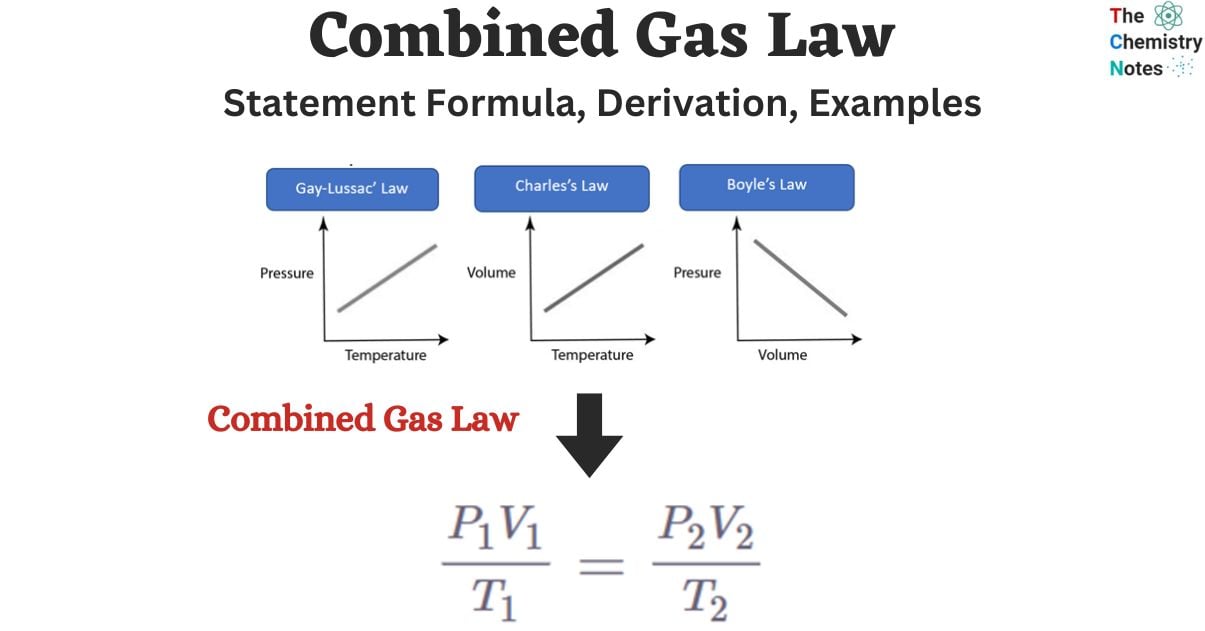
Combined Gas Law: Formula, Derivation, Examples

Ideal Gas Law - Ideal Gas Equation, Derivation, Solved Examples

Gay-Lussac's Law: Statement, Formula, Explanation, Example & FAQs

Partial pressure - Wikipedia
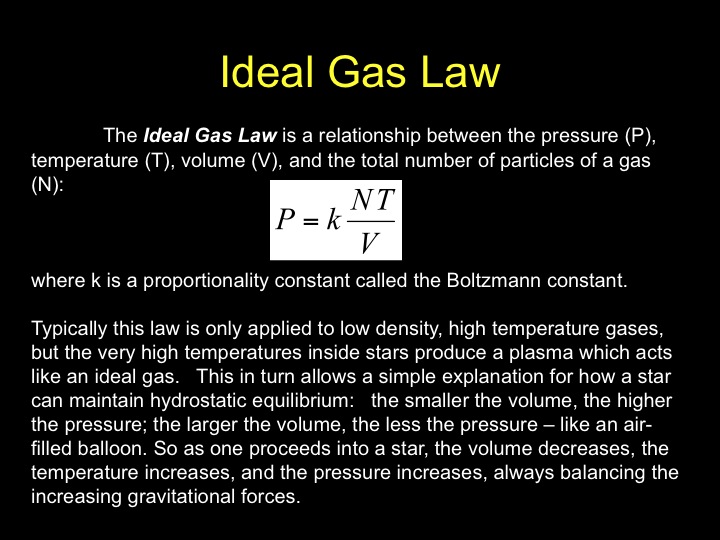
Ideal Gas Law