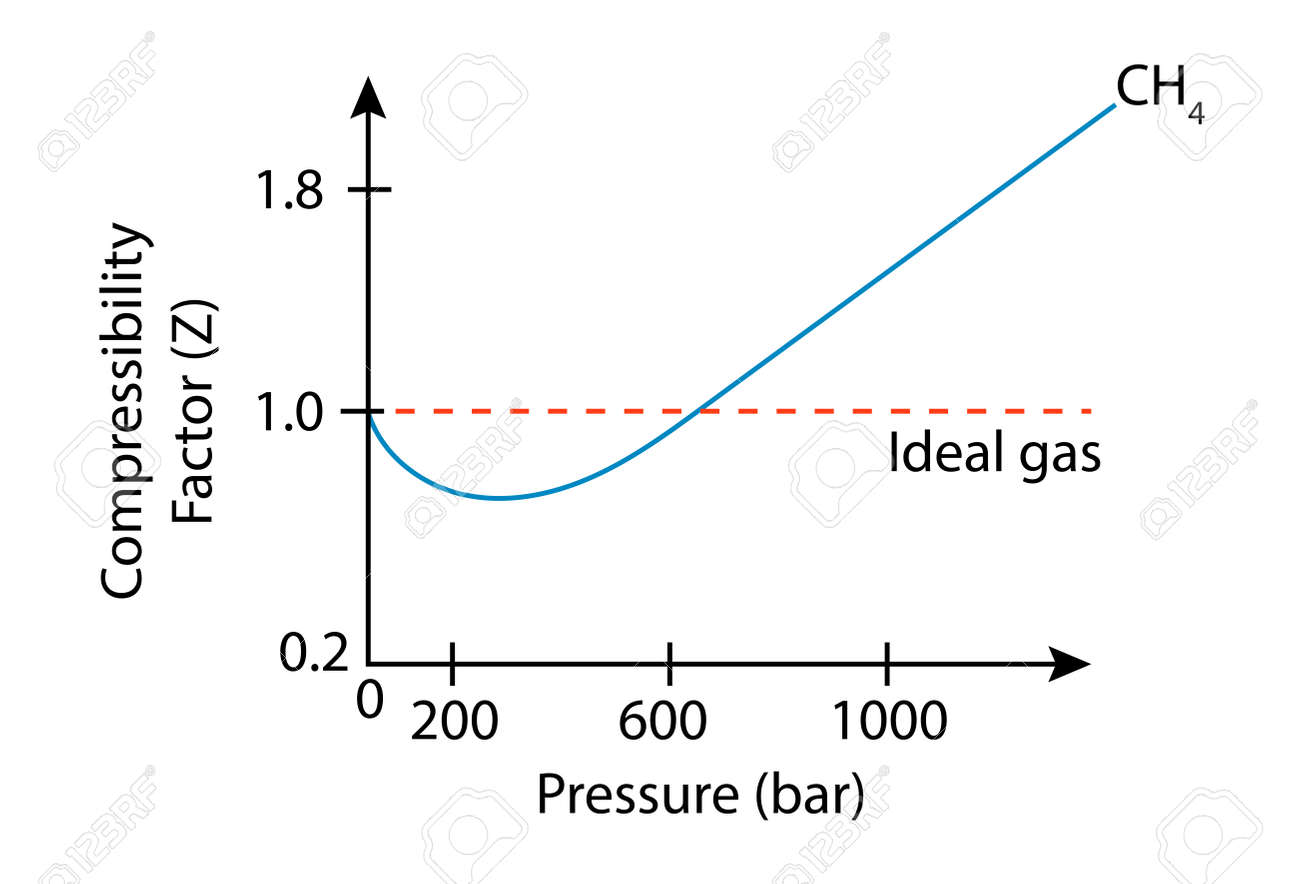
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

Modus Operandi for Practice: A Comprehensive Study Package on Atomic Structure and the Gaseous State, PDF, Spectroscopy
Thermo] Derivation of compressibility factor vs reduced pressure

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download
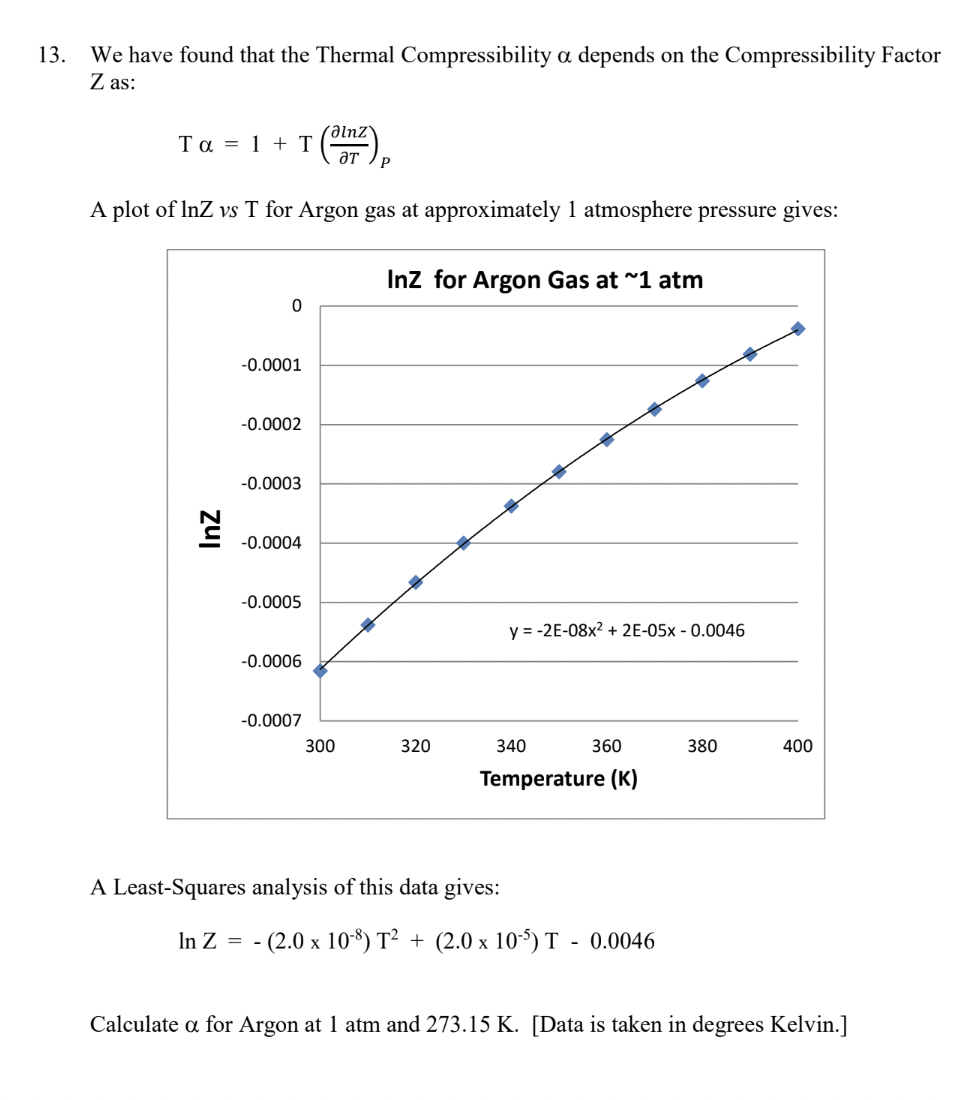
Solved 13. We have found that the Thermal Compressibility α

Non-Ideal Gas Behavior Chemistry: Atoms First

Kvpy 2016 SB - SX Stream PCM, PDF, Electron

Compressibility factor - Wikipedia

The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

2nd Year Holiday Assignment, PDF, Tangent

Modus Operandi for Practice: A Comprehensive Study Package on Atomic Structure and the Gaseous State, PDF, Spectroscopy

TT 4026 If the slope of' Z' (compressibility factor) v/s' P' curve is constant (slope = 109 atm?) a particular temperature (300K) and very high pressure, then calculate diameter of the molecules. (
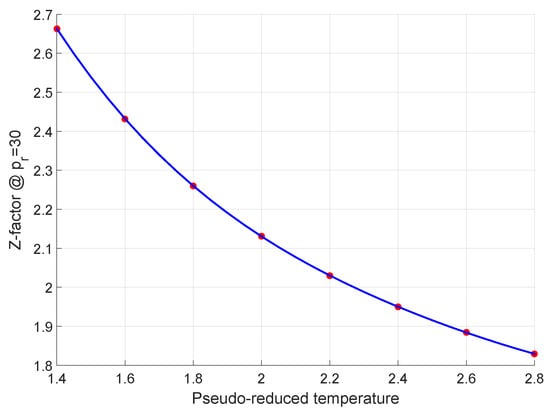
Energies, Free Full-Text