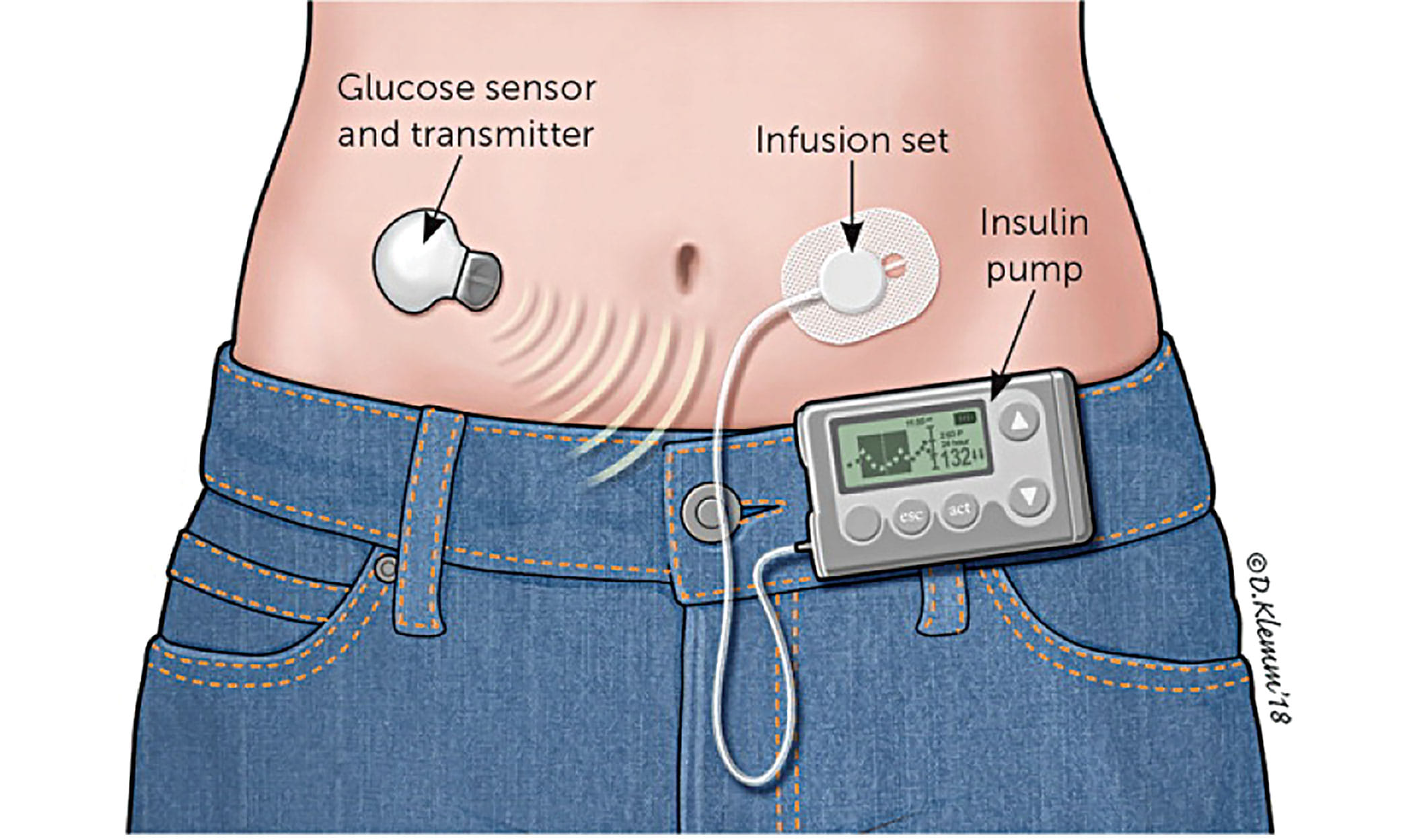
The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Medtronic - Wikipedia

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Medtronic pain pump recall deemed Class I - MassDevice

Report: Medtronic in negotiations to buy Israel-based insulin pump

MassDevice on LinkedIn: Butterfly Network to bring Ultrasound-on

The FDA Is Recalling Medtronic Insulin Pumps Over Hacking Concerns

Shareholder lawsuit over BD's Alaris pumps recall moves forward - MassDevice

Medtronic touts 'closed-loop' insulin management system

FDA Warns Hackers Could Hijack Medtronic MiniMed Insulin Pumps And
Medtronic MiniMed Insulin Pump Lawsuit & Recall

Eitan Medical's Sapphire infusion pump recall is Class I

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

The worst catheter-based device recalls of 2020 - MassDevice