
Most real gases depart from ideal behaviour at deviation from low temperature high pressure.
High positive potential energy (little separation) Repulsive interactions Intermediate separations attractive interactions dominate Large separations (on the right) the potential energy is zero and there is no interaction between the molecules..
Real gas molecules do attract one another (P id = P obs + constant) Real gas molecules are not point masses (V id = V obs - const.)
V id = V obs - nb b is a constant for different gases P id = P obs + a (n / V) 2 a is also different for different gases Ideal gas Law P id V id = nRT
Critical temperature (T c ) - the temperature above which a gas cannot be liquefied Critical pressure (P c ) – the minimum pressure that needs to be applied at T c to bring about liquefaction
For a perfect gas, the slope is zero Boyle temperature the slope is zero and the gas behaves perfectly over a wider range of conditions than at other temperatures.
Boyle temperature - for a van der Waal s gas, the Boyle temperature (T B ) is written
The reduced state variables are defined
Re-write the Van der Waals in terms of reduced variables
The chemical potential of a real gas is written in terms of its fugacity
In gaseous systems, we relate the fugacity (or activity) to the ideal pressure of the gas via.
Define the fugacity coefficient = f / P For a real gas.
Comparing the chemical potential of the real gas to the chemical potential of an ideal gas at the same pressure
The fugacity coefficients are obtained from the compression factors (Z) as shown below

JEE Main 2024 Syllabus : Download NTA JEE Main Syllabus for

The road ahead reaches a turning point in 2024

PPT - 1. Introducing the Ancient Debate: The Ideal versus the Real PowerPoint Presentation - ID:1707393

A non ideal gas follows the equation
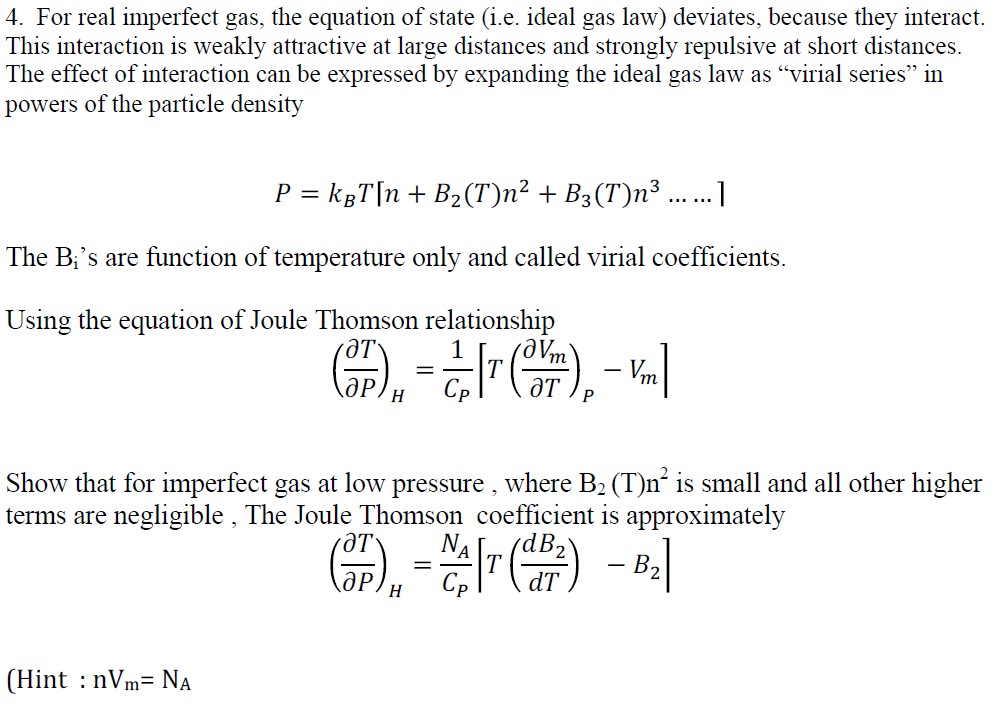
Solved For real imperfect gas, the equation of state (i.e.
RealClimate: The AMOC: tipping this century, or not?
PPT - Chemistry 231 PowerPoint Presentation, free download - ID:1431197

Chem Ch 16/#2 Today's To Do List l Equations of State catchup l More about the Critical State l Law of Corresponding States l Virial Equations l. - ppt download

Entropy, Free Full-Text

Real gas 1.molecules not always in motion (condense phase can be formed) 2.molecular size is non-negligible (there is molecular repulsion) 3.Molecules. - ppt download
GASES. - ppt download

Real Gases Deviation from ideal gas Law: -Gas particles have volume - Attraction exists between gas particles (liquefication) Ideal gas law applies only. - ppt download
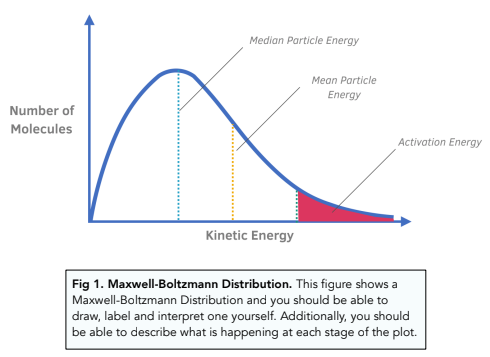
Kinetics - The Maxwell–Boltzmann Distribution and Catalysts (A







