An ideal gas is taken from (Pi , Vi ) to (Pi , Vi ) in three different ways. Identify the process in (d) Equal work is done in Process A, B & C

One mole of an ideal gas is taken from state A to state B by three different processes a A C B, b A D B and c A E B as

SOLVED: 3P; Pi A V 3V; A 1 mole of ideal gas initially at Pi-l Pa, Vi–5 m, and Ti= 0°C is taken through a cycle as shown in the above Figure.
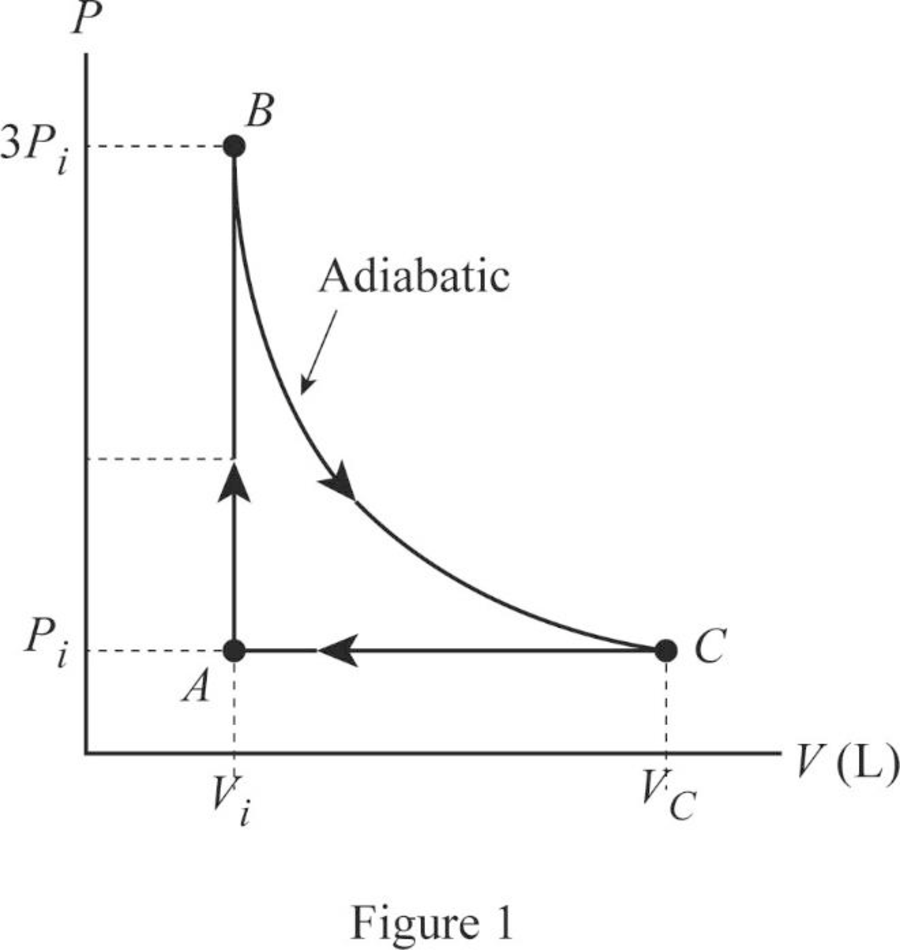
An ideal gas with specific heat ratio γ confined to a cylinder is put through a closed cycle. Initially, the gas is at P i , V i , and T i .

An ideal gas is taken from state A to the state B, as shown in the P V diagram. The work done in the process is
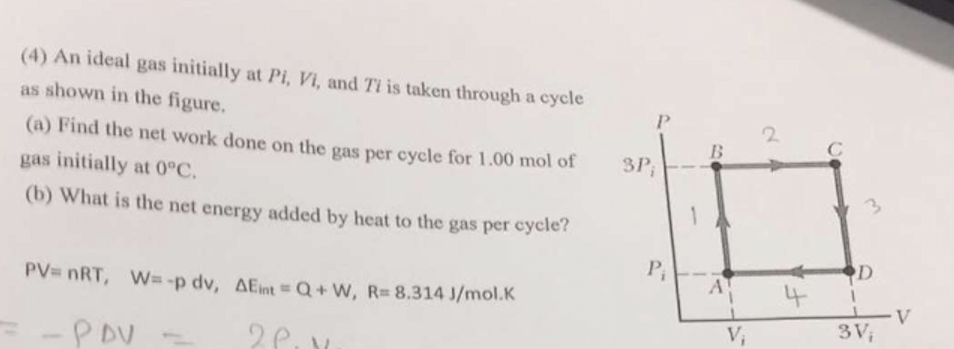
Solved (4) An ideal gas initially at Pi, Vi, and Ti is taken

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and

An ideal gas goes from state A to state B via three different processes as indicated in the P V diagram. If Q 1, Q 2, Q 3 indicate the heat absorbed

An ideal gas is taken around the cycle ABCA as shown in P-V diagram. The net work done by the ga
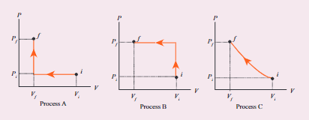
An ideal gas is taken fromPiVitoPfVfin three different ways Identify the process in which the work done on the gas the most

An ideal gas is taken from state A to state B via three different processes as shown in the pressure volume (P-V) diagram. If Q4, Q, & Q, indicates the heat absorbed

An ideal gas which has `gamma = 4//3` is taken from A to B according to the diagram

An ideal gas is taken around the cycle ABCA as shown in P-V diagram.The net work done by the gas during the cycle is equal to

Solved An ideal gas initially at Pi, Vi, and Ti is taken
An ideal gas is taken from (Pi , Vi ) to (Pi , Vi ) in three different ways. - Sarthaks eConnect

An ideal gas is taken from state A Pressure P, Volume V to the state B Pressure P/2, Volume 2V along a straight line path in PV diagram as shown in the