
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

20 dm^(3) of SO(2) diffuse through a porous partitions in 60 second Wh

3) 20%, 35% (4) 66.67%, 15% under sure is 37 At 37°C and 0.1 atm, the mass density of phosphorous vapour is 0.5 g/L. The molecular formula of phosphorus is take R =

The compressibility factor (Z) of one mole of a van der Waals' gas

At a high pressure, the compressibility factor (Z) of a real gas is us

Telugu] The compression factor (compressibility factor) for one mole

Poulduly 59. What is the compressibility fac is the compressibility factor (Z) 0.02 mole co Vanderwaals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. . RT =

Graph depicting correct behaviour of ideal gas and H(2) gas will be (n

Filo Student Questions For CBSE , Grade 9
Filo Student Questions For CBSE , Grade 9
Why does CH4 have a greater value of van der Waals' constant than
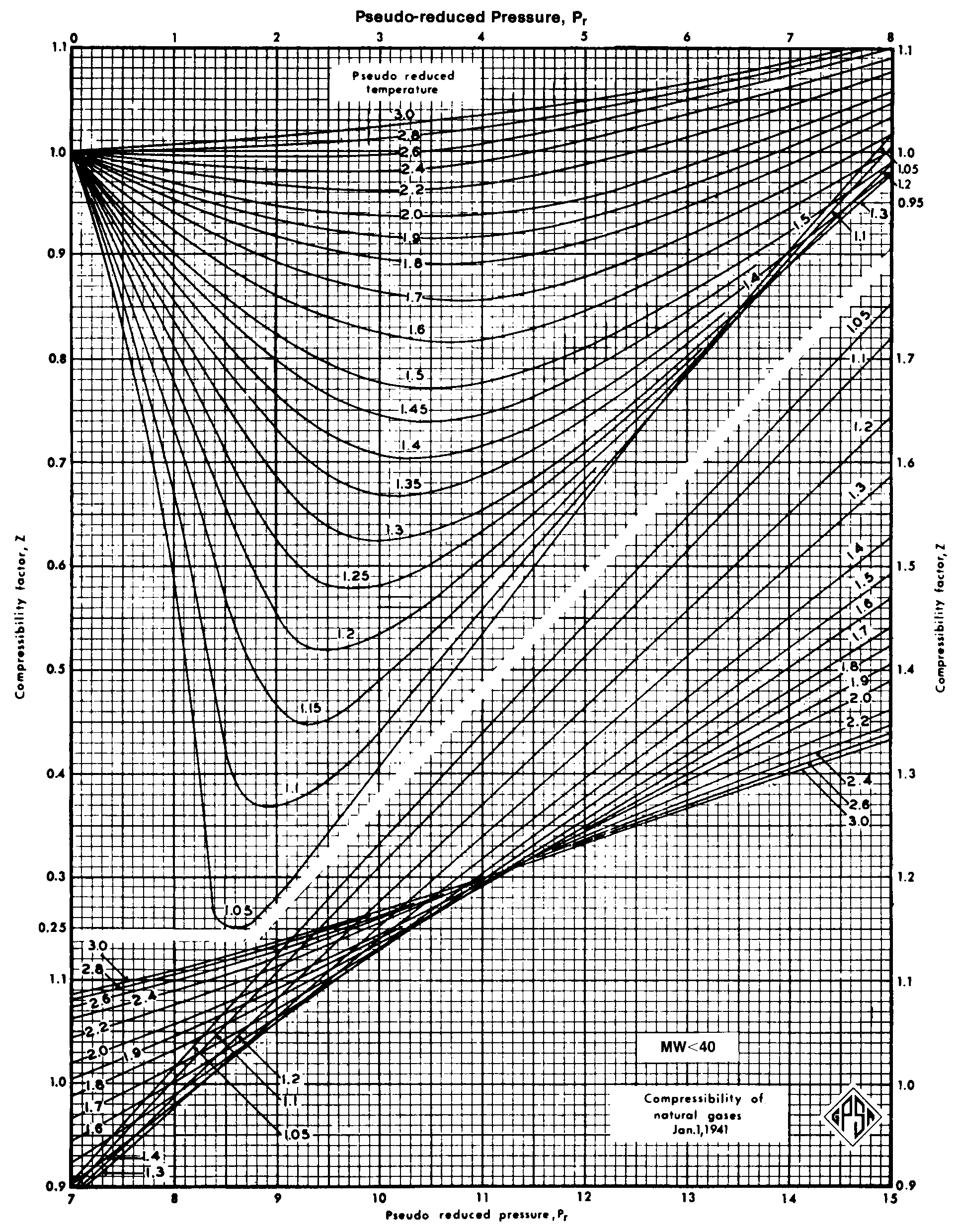
Determine Compressibility Factor, Z Factor - Engineering Units

DPP No. : 21 Total Marks : 40 Max. Time : 10mln Single choice Objective (..







