
Which of the following statements is/are correct? (a) all real gases are less compressible than ideal gas at high pressures? (6) hydrogen and helium are more co

Which of the following statements is(are) true? For the fals

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF
Solved Question 21 Which of the following statements

Deviation Of Real Gas From Ideal Gas Behavior
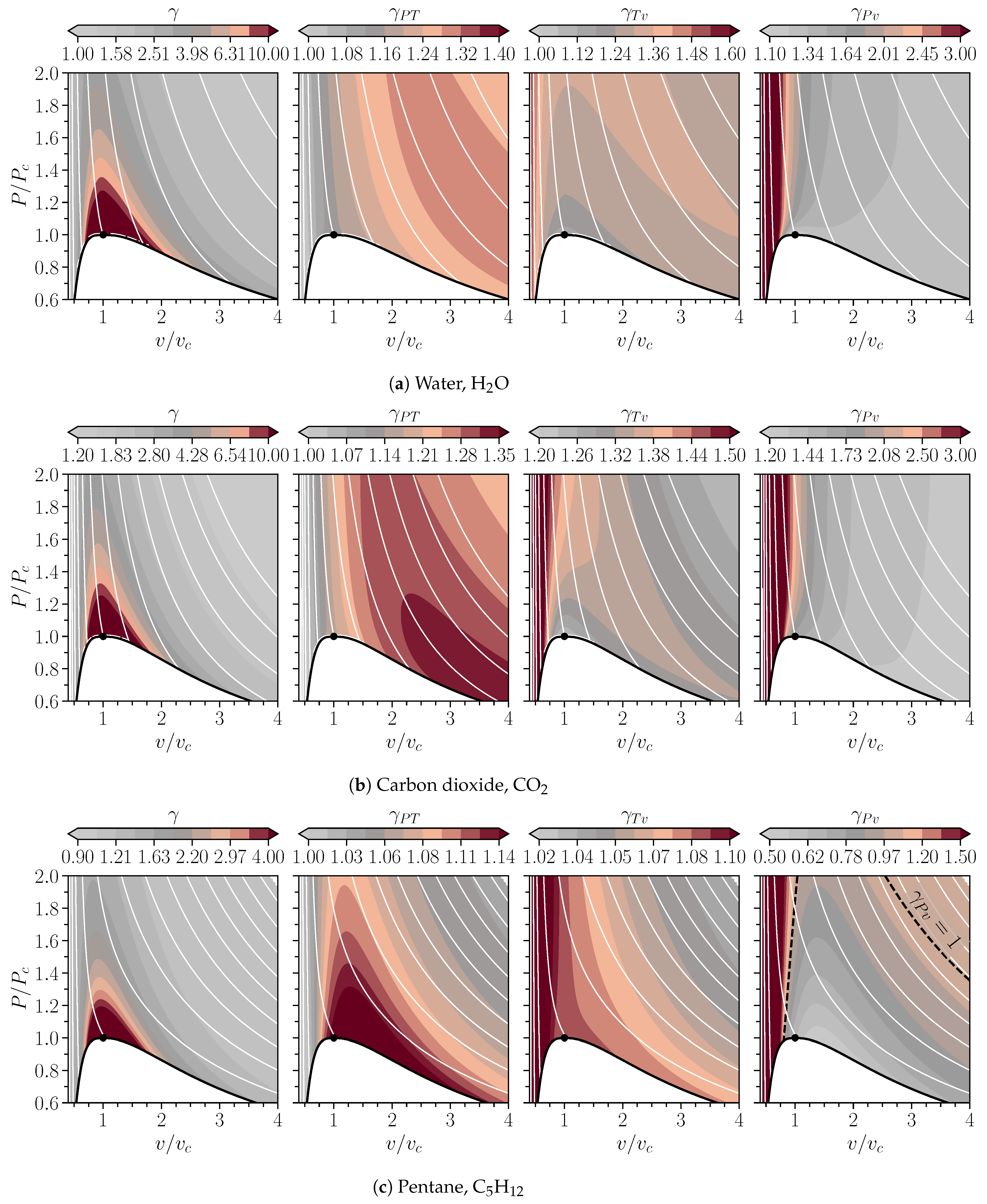
Energies, Free Full-Text

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF

Compressibility factor (z): real gases deviate from ideal behav-Turito
Ideal fluids, bernouilli's law

SOLVED: Make judgment of the following statements and justify your

How to Reduce Smart Contract Gas Usage

Liquid - Wikipedia

Consider the equation, Z=dfrac{PV}{nRT}. Which of the following statements is correct?When Z>1, real gases are easier to compress than the ideal gasWhen Z>1, real gases are difficult to compressWhen Z=1, real gases






