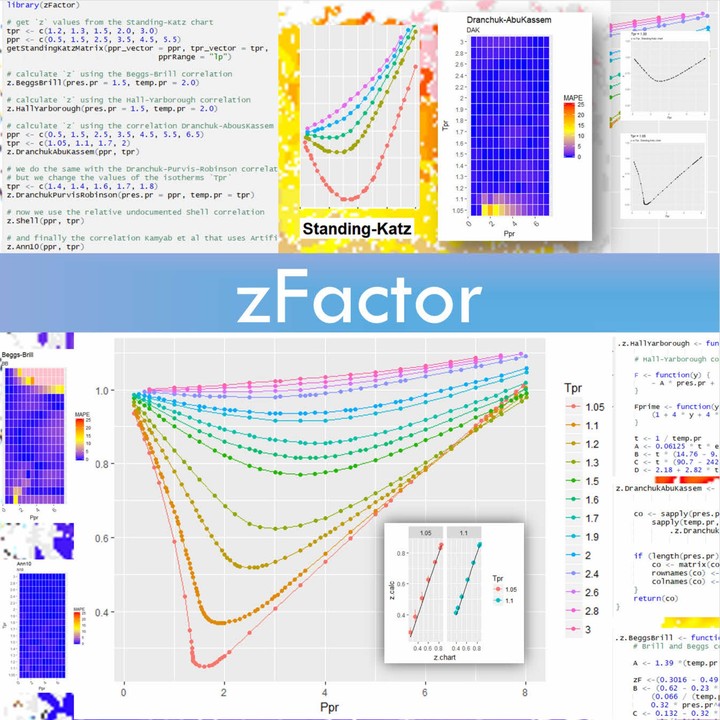
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
Gas Laws – First Year General Chemistry

Compressibility Factor of Gas Overview, Equation & Chart
At Critical Temperature,pressure and volume . The compressibility

Sound, Properties, Types, & Facts

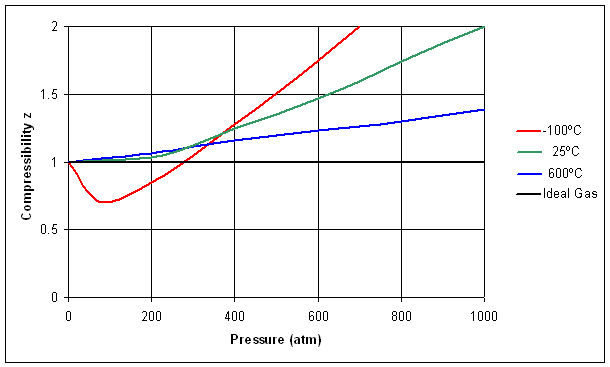
The compression factor (compressibility factor) for `1 mol` of a

Physical Chemistry The Compression Factor (Z) [w/1 example

physical chemistry - Compressibility Factor Graph - Which gas attains a deeper minimum? - Chemistry Stack Exchange

Physical Chemistry The Compression Factor (Z) [w/1 example

Real Gases and Compressibility Factor
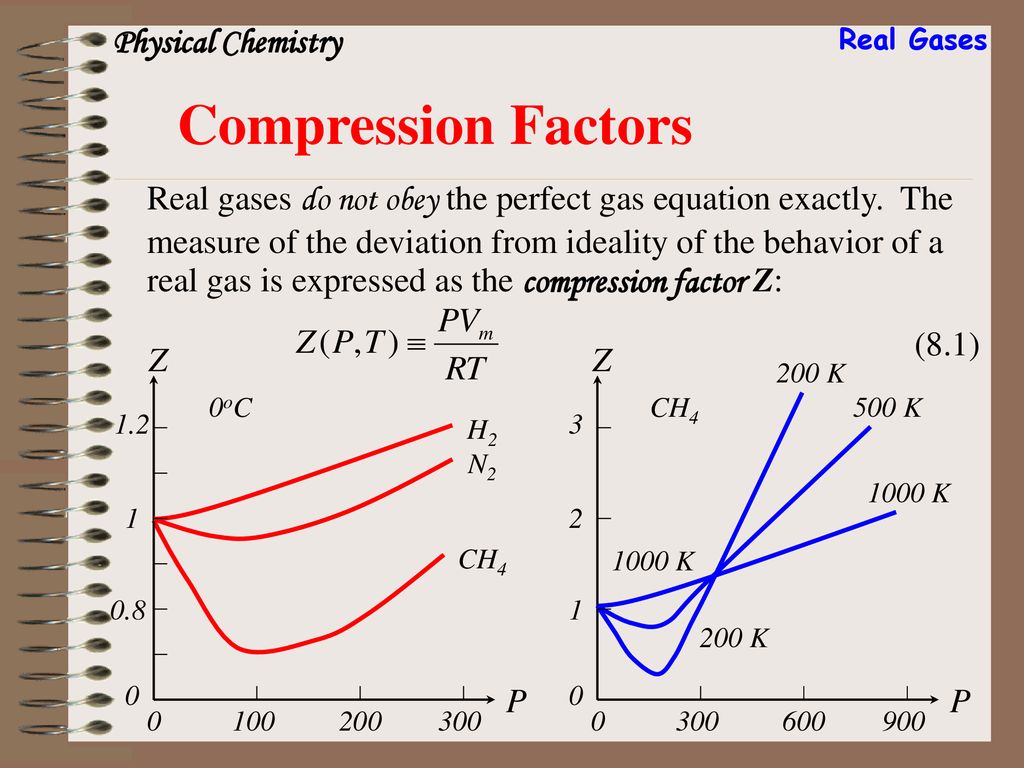
Chapter 8 Real Gases. - ppt download

Van der Waals equation - Wikipedia

physical chemistry - Why do some gases have lower value of Z for a

Physical Chemistry The Compression Factor (Z) [w/1 example
The periodic table and the physics that drives it