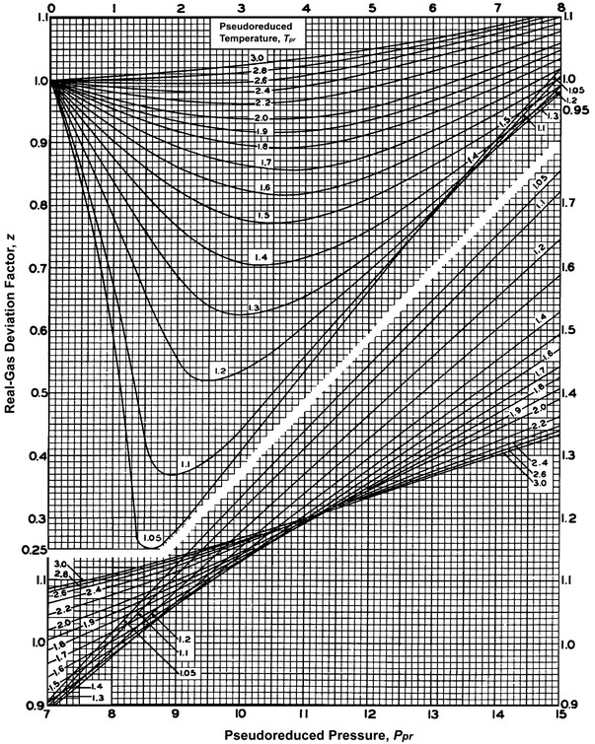
Miscellaneous Contributions by: - ppt video online download

Statement-1. Compressibility factor of non-ideal gases is always less than1. Statement-2. Non-id

Determine Compressibility of Gases

Compressibility factor - Wikipedia

The compressibility factor of a gas is less than 1 at STP. Its molar volume Vm will be

Compressibility factor (gases) - Knowino

Explain how the compression factor varies with pressure and

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1 -displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
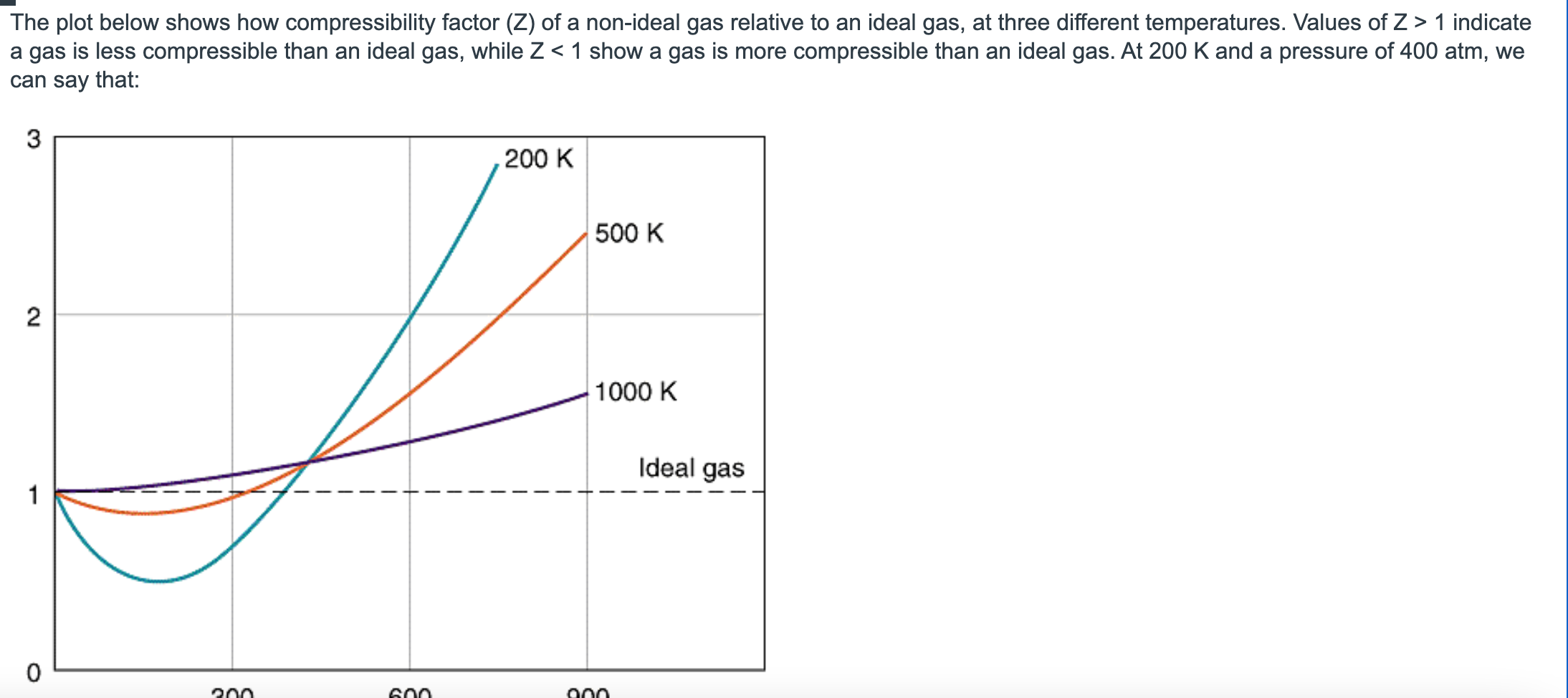
Solved The plot below shows how compressibility factor (Z)
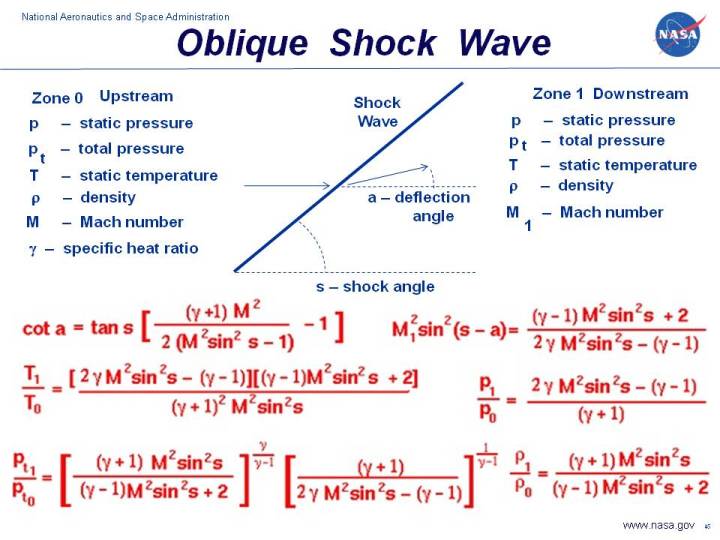
Oblique Shock Waves

where Z is the compressibility factor that