

gaseous state

al Gases f.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given: RT =

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Flow‐through drying of porous media - Mahadevan - 2006 - AIChE Journal - Wiley Online Library

Van Der Waals Equation - an overview
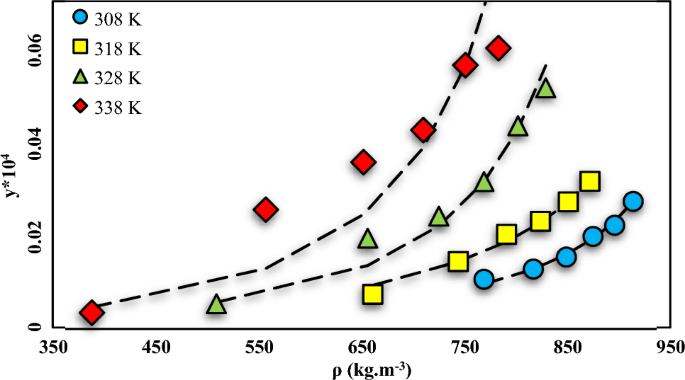
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2

Real Gases Introductory Chemistry

jo 22] What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible.
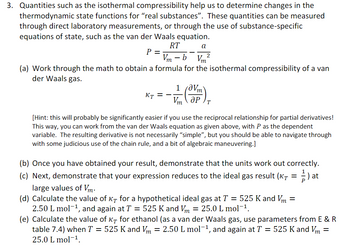
Answered: Quantities such as the isothermal…
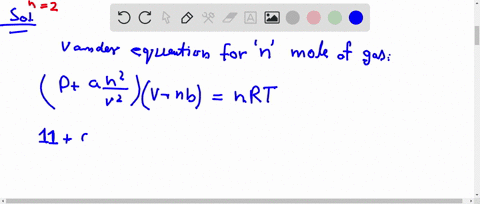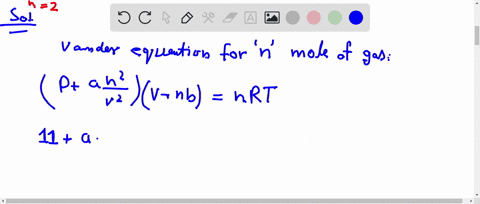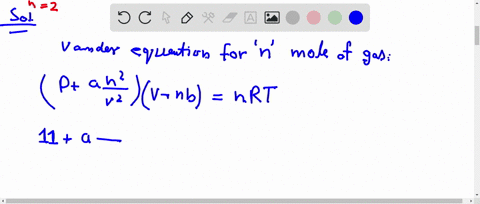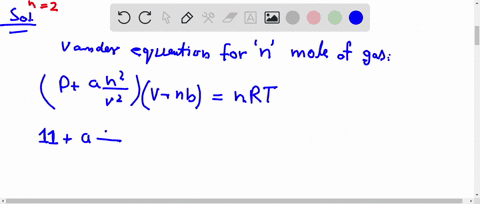
⏩SOLVED:The compression factor (compressibility factor) for one mole…

Compressibility factor - Wikipedia

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange

Objectives_template





