200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

4.64 A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate
What is the mass of a 75% pure CaCO3 sample which on heating gives 2.2 g CO2 with 50% yield? - Quora

Thermochemical energy storage system development utilising limestone - ScienceDirect
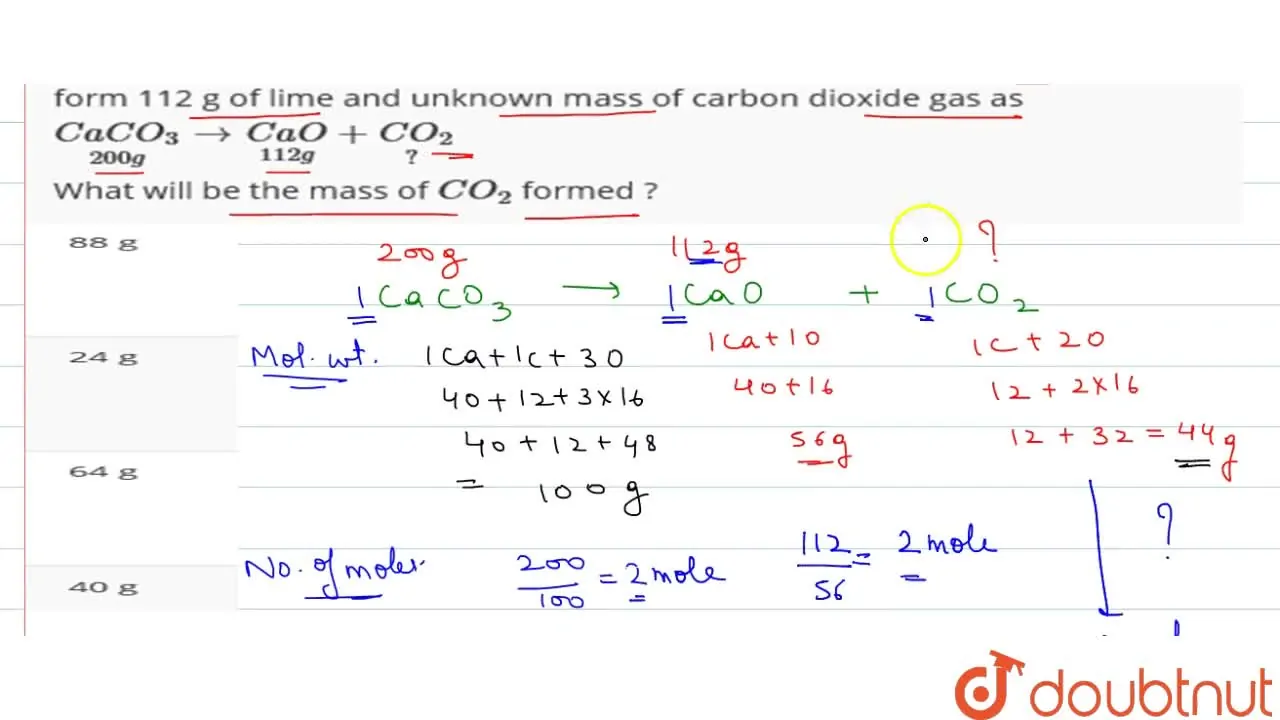
When 200 g of lime strongly heated , it undergoes thermal decompositio

MC, PDF, Phase (Matter)

Alteration in molecular structure of alkali activated slag with various water to binder ratios under accelerated carbonation

Cement testing

1996 2009 Kcse Chemistry 1, PDF, Chlorine

Mass if `CO_(2)` Produced on heating 20g of 40% pure limestone :-

Thermochemical energy storage system development utilising limestone - ScienceDirect







