Share
Inorganic Acids and Bases - pKa Values

Equilibrium - Flip eBook Pages 51-100

The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo
CHEM 245 - Acid-base chemistry

SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [
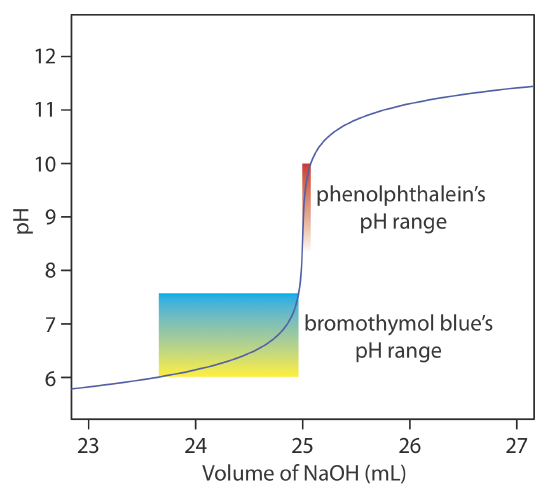
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts

PHARMACEUTICAL ANALYSIS I - ACID BASE TITRATIONS

PDF) Quantitative Chemical Analysis
Related products

Model BH - Classic Black Leather Motorcycle Jacket - House Jacket with SLV Stripes

Tolerância zero: Sumob fiscaliza ônibus na região do Barreiro em BH. Pelo menos quatro coletivos foram recolhidos - Rádio CDL FM - 102,9

BHB Complete, Beta-Hydroxybutyrate, 120 Capsules

BH Butting (Body Hook Butting) - Seam School - Craft Seaming
You may also like



