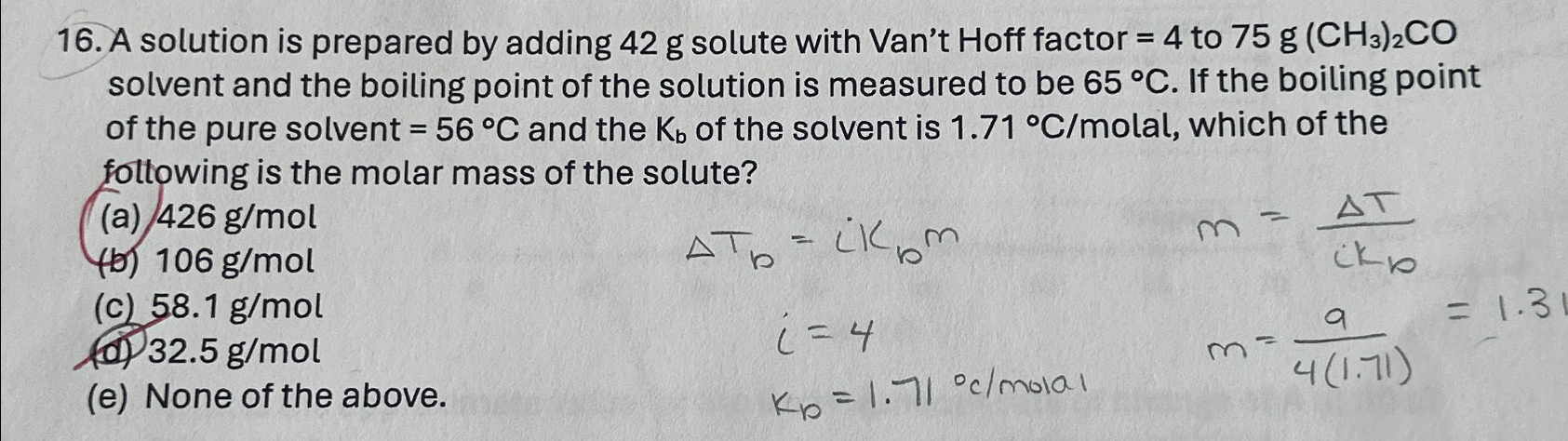Share your videos with friends, family, and the world
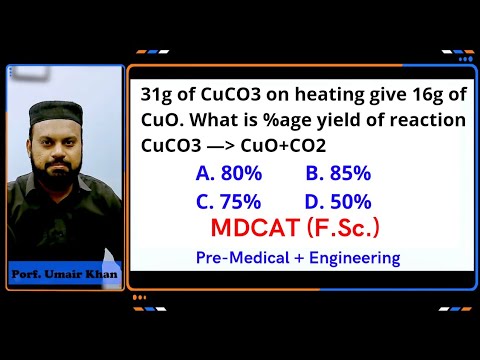
31g of CuCO3 on heating give 16g of CuO. what is %age yield of reaction. 80% 85% 75% 50%

Limiting Reaction Calculations Practice Flashcards

If 10 g of H2. reacts with 42 g of N2, ther identify the correct statem..

42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g

Limiting Reaction Calculations Practice Flashcards
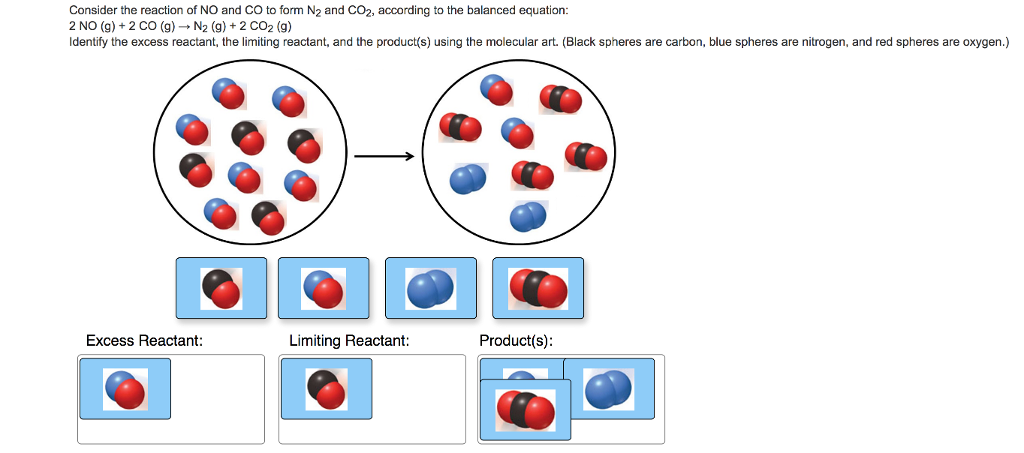
Solved Consider the reaction of NO and CO to form N2 and

Empirical formula of a hydrocarbon having 80% C and 20% of hydrogen is a.CH b.CH3 c.CH2 d.CH4 MDCAT
28g of N2 and 10g H2 are combined to form ammonia.identify limiting reagent and composition of mixture
How to calculate the maximum mass of ammonia, NH3, that could be made from 42 tonnes of nitrogen and excess hydrogen - Quora
How to justify oxidant and reductant in the following reaction N2+O2…2NO - Quora

Nitric oxide (NO) reacts with oxygen gas to form nitrogen di
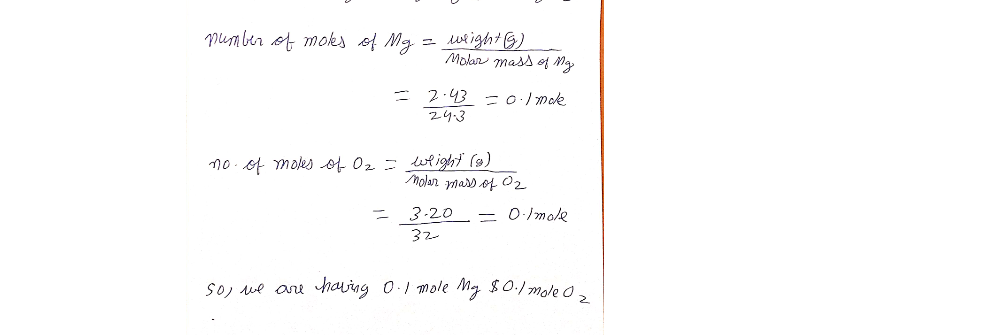
Answered: Suppose 2.43 g of magnesium is reacted…
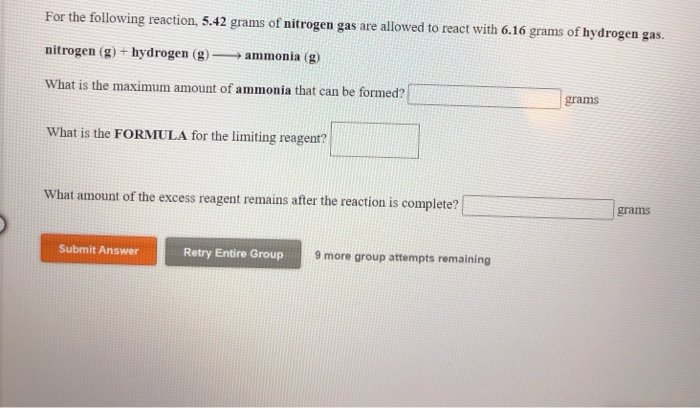
Solved For the following reaction, 10.9 grams of nitrogen

Answered: LIMITING REAGENTS TO2/S21 Based on the…