
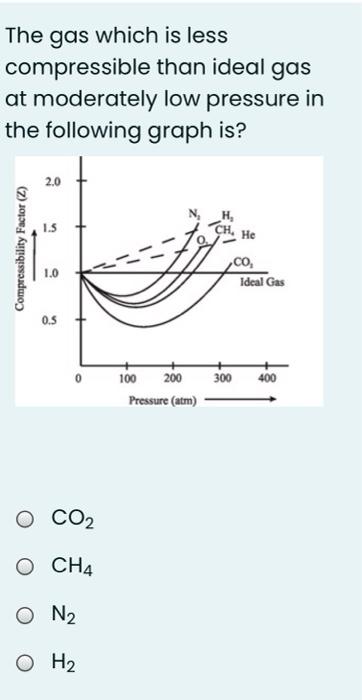
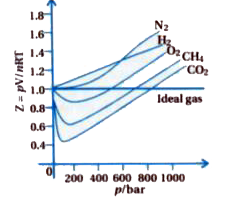
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

The graph of compressibility factor (Z) vs. P for one mole of a real g
Solved The first order rate constant of the decomposition of
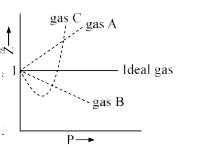
Gas C is a real gas and we can find 'a' and 'b' if intersection data i

Determine Compressibility of Gases
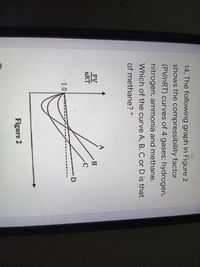
Answered: 14, The following graph in Figure 2…
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora

SOLVED: Use the graph of compressibility factors in terms of reduced variables to answer the following about methane (pc = 4.595 MPa, Tc = 190.55 K, and M = 16.04 g/mol): a)

In the following compressibility factor (Z) versus pressure graph at 300 K, the compressibility of CH4 at pressure < 200 bar deviates from ideal behaviour because 
KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download

47. In the following compressibility factor (Z) vs pressure graph 300 K, the compressibility factor of CH4 pressures < 200 bar deviates from ideal behavior because

Gas Compressibility - an overview
The graph of compressibility factor (Z) vs. P for one mole of a

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted as a
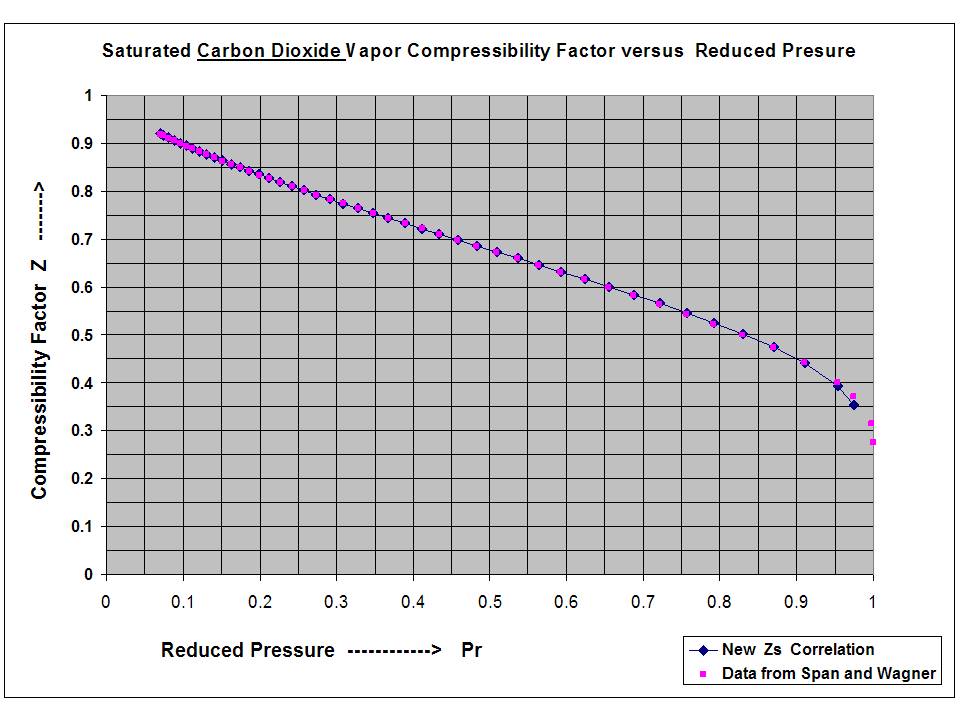
New compact Equations for the Compressibility Factor Z and Density of Liquid and Vapor Carbon Dioxide
Gujrati] What is compressibility factor (Z) ? (i) deviation facto







