

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download

Acentric Factor - an overview

Compressibility factor - Wikipedia
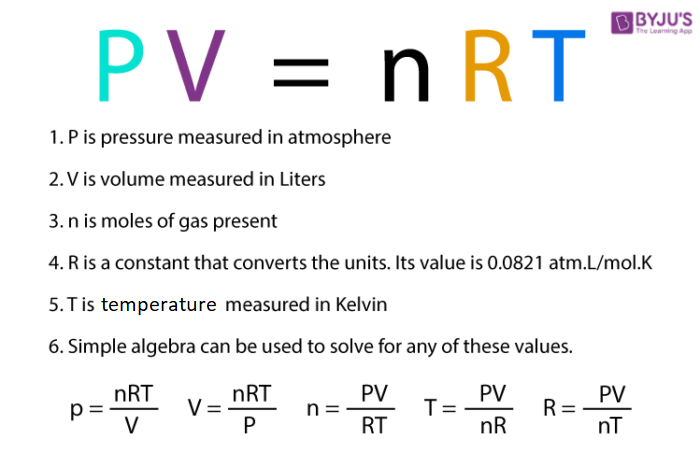
Ideal Gas Law Equation Compressibility Of Natural Gas - Chemistry

6.3: Van der Waals and Other Gases - Physics LibreTexts
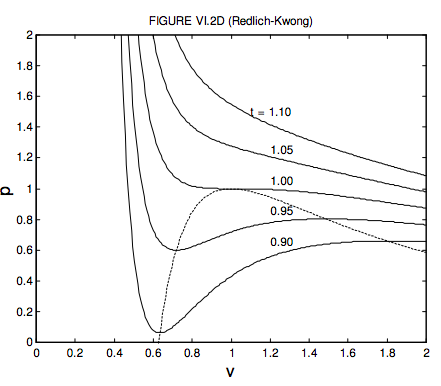
6.3: Van der Waals and Other Gases - Physics LibreTexts
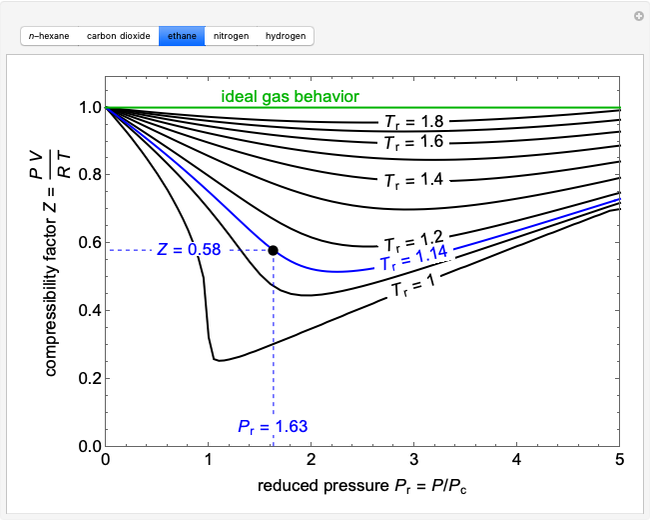
Compressibility Factor Charts - Wolfram Demonstrations Project

Real gas 1.molecules not always in motion (condense phase can be formed) 2.molecular size is non-negligible (there is molecular repulsion) 3.Molecules. - ppt download

Mechanisms of Myocardial Ischemia in Cancer Patients: A State-of-the-Art Review of Obstructive Versus Non-Obstructive Causes

Physical Chemistry The Compression Factor (Z) [w/1 example]
Ethylbenzene (CAS 100-41-4) - Chemical & Physical Properties by Cheméo

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Compressibility factor (z): real gases deviate from ideal behav-Turito






