
Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

Compressibility factor (z): real gases deviate from ideal behav-Turito

Compressibility factor - Wikipedia

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor - Wikipedia

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Gas Compressibility - an overview

Non-Ideal Gas Behavior Chemistry: Atoms First

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Description of real gases: Compression factor

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a

Compressibility factor (gases) - Citizendium

COMPRESSIBILITY FACTOR
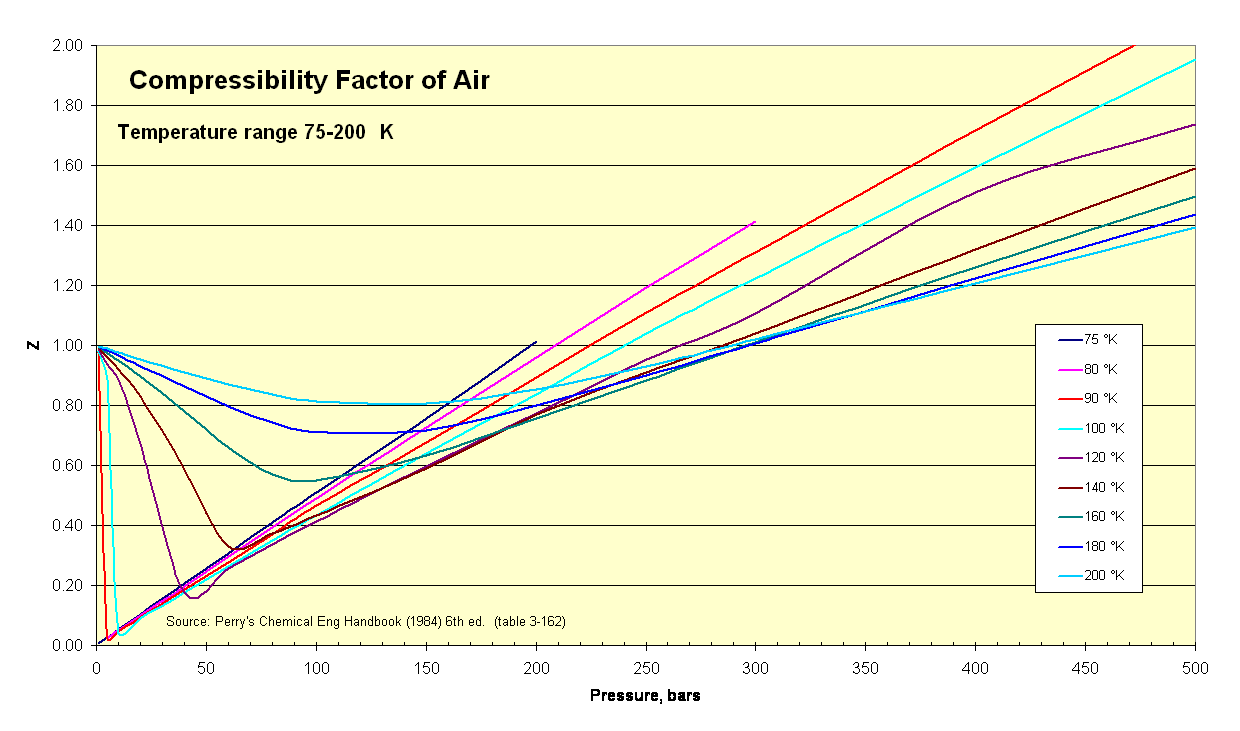
Air Compressibility Factor Table - EnggCyclopedia

Virial coefficients: empirical approx. of the compression factor

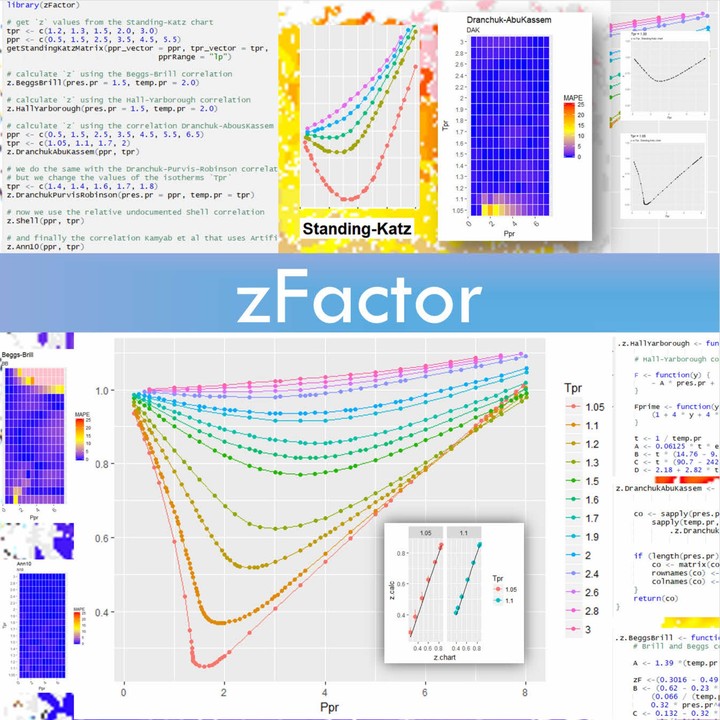



)

