At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

If Z is compressibility factor, vander Waals equation low pressure

SOLVED: I need the answer as soon as possible. 16. If Z is a

Van der Waals equation, when pressure correction is ignored, one
Why is p'=an^2/V^2 in van der waal's equation? - Quora

physical chemistry - Why do some gases have lower value of Z for a
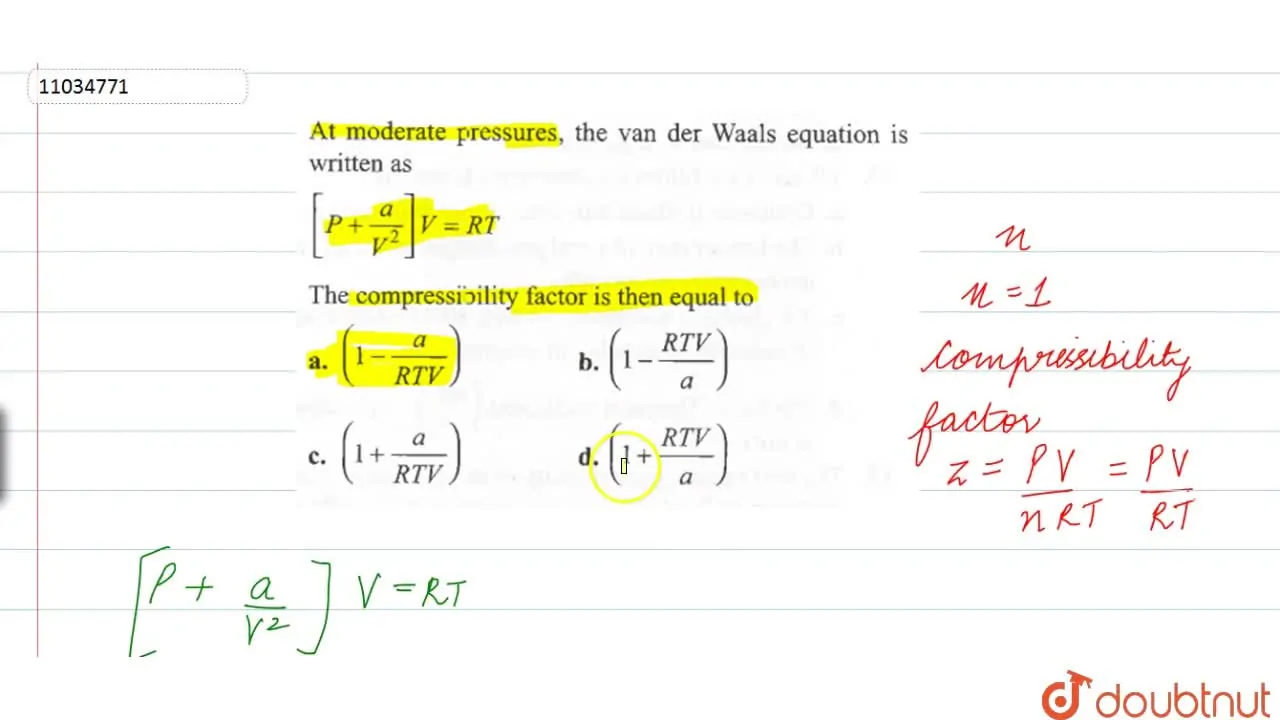
At low pressures, the van der Waals equation is written as [P+(a)/(V^(
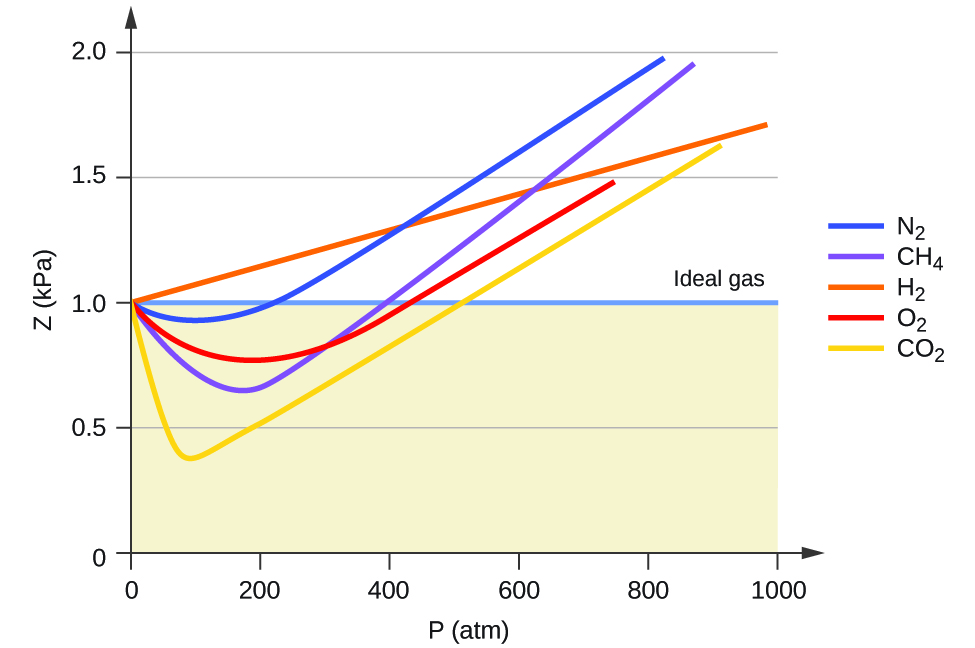
12.6 Non-Ideal Gas Behaviour – Enhanced Introductory College Chemistry
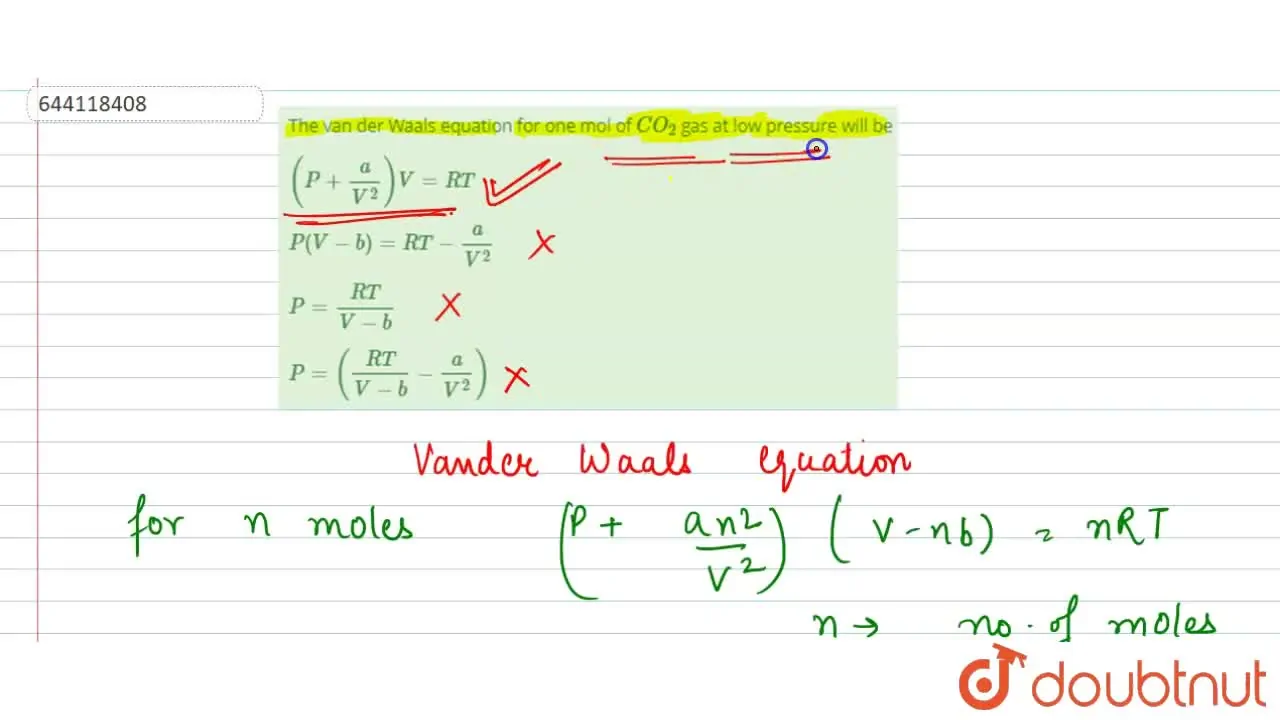
The van der Waals equation for one mol of CO(2) gas at low pressure wi
What is the unit of a and b in van der Waals' equation if it is

Fugacity, Activity, Thermo Graphs, PDF, Gases

For real gases, van der Waals' equation is written as `(P+(an^(2

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0

Bengali] At a low pressure, the van der waals equation reduces to (P+





