Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T2. The final pressure pf is :
Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T2- The final pressure pf is -
Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after the temperature increase.
Hence in the given c

Two closed bulbs of equal volume (V) containing an ideal gas initia

Two closed bulbs of equal volume (V) containing an ideal gas initially pressure p and temperature T. are connected through a narrow tube of negligible volume as shown in the figure below.

Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure
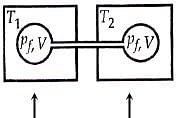
Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

Welcome to Chem Zipper.com: Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure Pi and temperature Ti are connected through a narrow tube of negligible volume as

Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and tem-perature T1 are con nected th rough a narrow tube of negligible volume as shown in
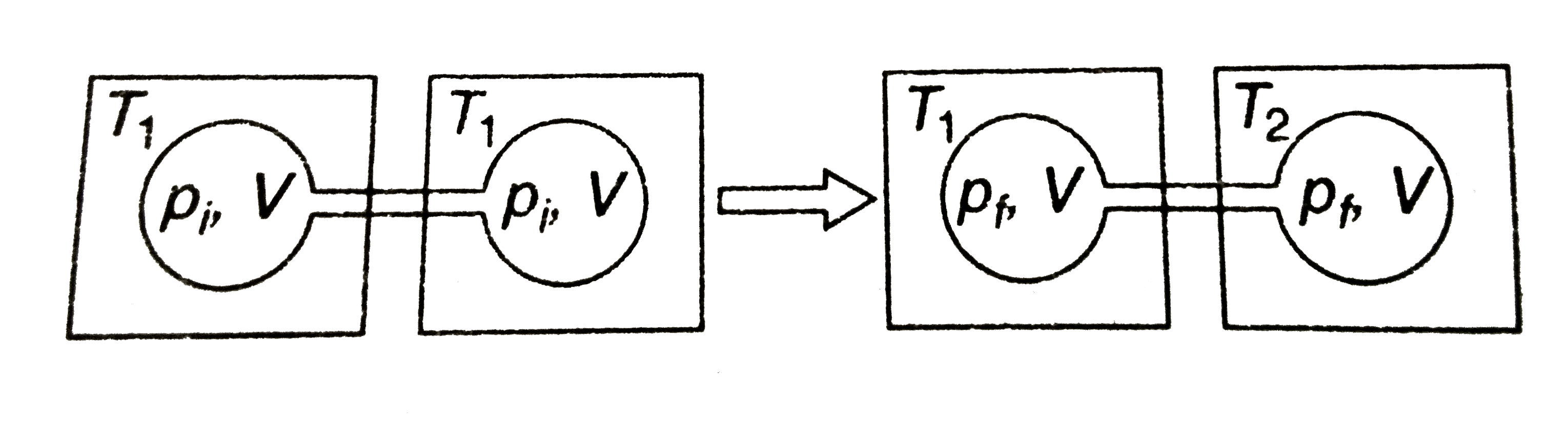
Two closed bulbs of equal volume (V) containing an ideal gas initially

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

2.ideal Gas - Final, PDF, Gases
Two bulbs A and B of same volume contain an ideal gas at temperature T and pressure P. When the temperature of the bulb B is doubled and that of A remains

Two closed bulbs of equal volume (V) containing an ideal gas initially
If a volume containing gas is compressed to half, how many moles of ga