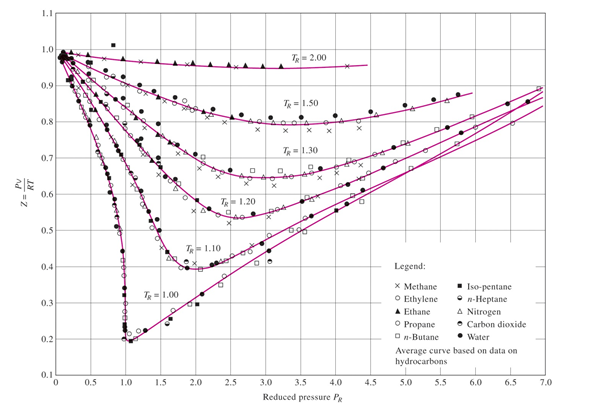
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Energies, Free Full-Text

If Z is a compressibility factor, van der Waals equation at low pressure ..

Compressibility Factor Calculator

SOLVED: physical chemistry Using the expressions for the critical constants in terms of the van der Waals constant, calculate the value of the compressibility factor at the critical state [Answer: 3/8]

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))
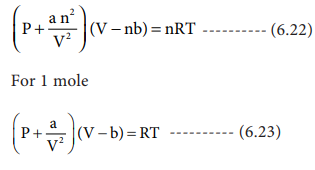
Derivation of critical constants from van der Waals constant

Van der Waals equation: van der Walls EOS, [Pr*3/Vr^2] [3Vr-1] =

Compressibility factor (gases) - Citizendium

Non-Ideal Gas Behavior Chemistry: Atoms First

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility factor (z): real gases deviate from ideal behav-Turito

6.3: Van der Waals and Other Gases - Physics LibreTexts

JEE: Van der Waals Equation, Chemistry By Unacademy